Fish Velvet Disease is a common affliction of freshwater fish. It is caused by a parasite that attacks the fish’s skin, fin and gill.
This disease is also known as “velvet,” “rust,” and “gold dust disease,” in reference to the iridescent sheen the disease gives to heavily affected fish.
The disease is most often seen in aquarium fish, but can also affect wild fish populations. Fish velvet is characterized by white, velvety growths on the fish’s skin.

Amyloodiniosis (Velvet Disease, Marine Velvet Or Rust Disease)
The dinoflagellate Amyloodinium ocellatum causes amyloodiniosis or marine velvet disease, which is among one of the most serious parasitic diseases affecting warmwater marine fish species culture worldwide.
Amyloodinium ocellatum, the agent to blame for amyloidosis (marine velvet, velvet disease), affects marine and brackish fish in a broad range of warm and temperate habitats.
Researcher recorded disease outbreaks with high morbidity and mortality rates in European seabass cultured in marine environments.
What Causes Velvet in Fish
The environmental conditions, including water temperature and salinity, were best suited that parasites might establish themselves, survive, and reproduce.
Brown (1931) first illustrated amylodium ocellatum, and according to him, this is among the most detrimental pathogens affecting the culture of lakes and seas. A similarly active organism, Oodinium sp. and Piscinoodinium spp. are found in freshwater fish.
Effect of Fish Velvet
Massive decline in fishery stock can occur due to the contributions of amyloid. They create severe loss in intensive high-density culture systems and have caused a long list of problems in public aquaria, aquaculture systems and at home.
For instance, red drum are known to be extremely vulnerable to this infection.
Susceptible Species
They are susceptible to a wide verities of fresh and salt water fishes. Amylodidium has been reported in more than 100 species in North America.
Geographical Distribution
The disease is very common in North America, Mediterranean sea, South Asia and Africa.
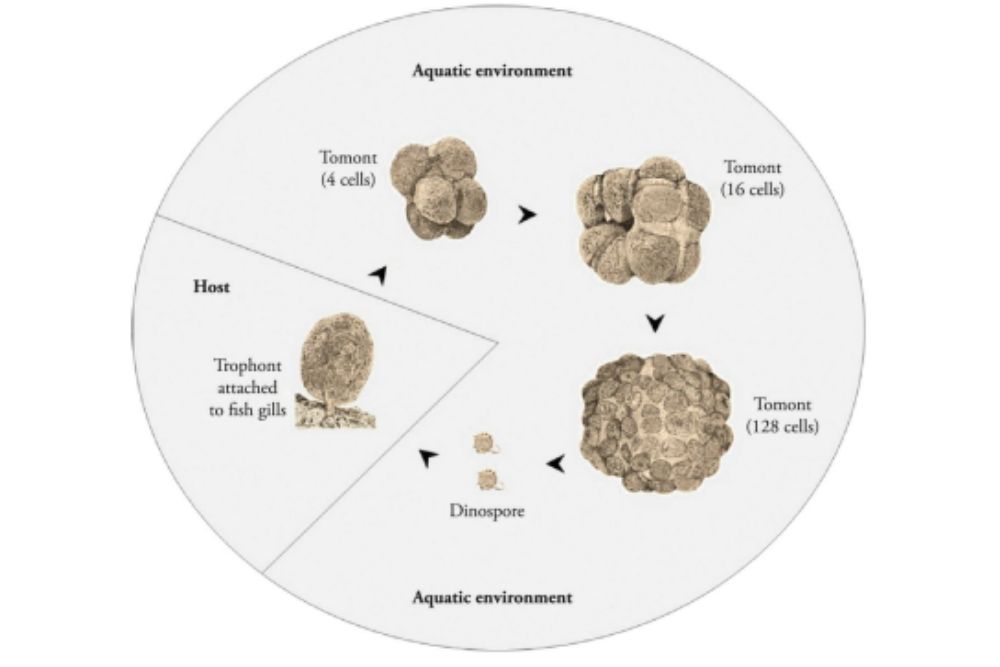
Life Cycle
The life cycle of amylodinium is very different from that of many other parasites of fish. Many single-celled parasites that infect the external surface of fish divide with their cells by sharing a chromosomal area through binary fission.
An adult organism divides into two, and each of those halves splits into two, and so forth.
The trophont adult stage merges into the fish, for producing next generation they slips to the water and forming cysts or tomont and waits there for an incipient cell differentiation.
The tomont undergoes multiple divisions depending on temperature and salinity.
Each tomont may produce up to 256 dinospores through continuous subsequent divisions, which can in turn contaminate fish.
The dinospores may remain inactive for approximately 15 days without the presence of a fish host. The dinospores that contribute to the spread of infections swim through the water until they attach themselves to a host.
Once find their appropriate host, they lose their flagella and attached to the host skin, fin or gill developing into the infective parasite stage.
Signs & Symptoms of Velvet in Fish
The parasite produces an powdery off-white velvety appearance on fish skin that have been affected by it.
Decreased feeding activity, flashing rubbing against the tank or against the bottom substrate, and coughing backflushing water across the gills are all signs of behavioral changes.
Wounded fish may have a dull golden or brown sheen on their skin. Microscopic examination of the skin may reveal scale and mucus accumulation.
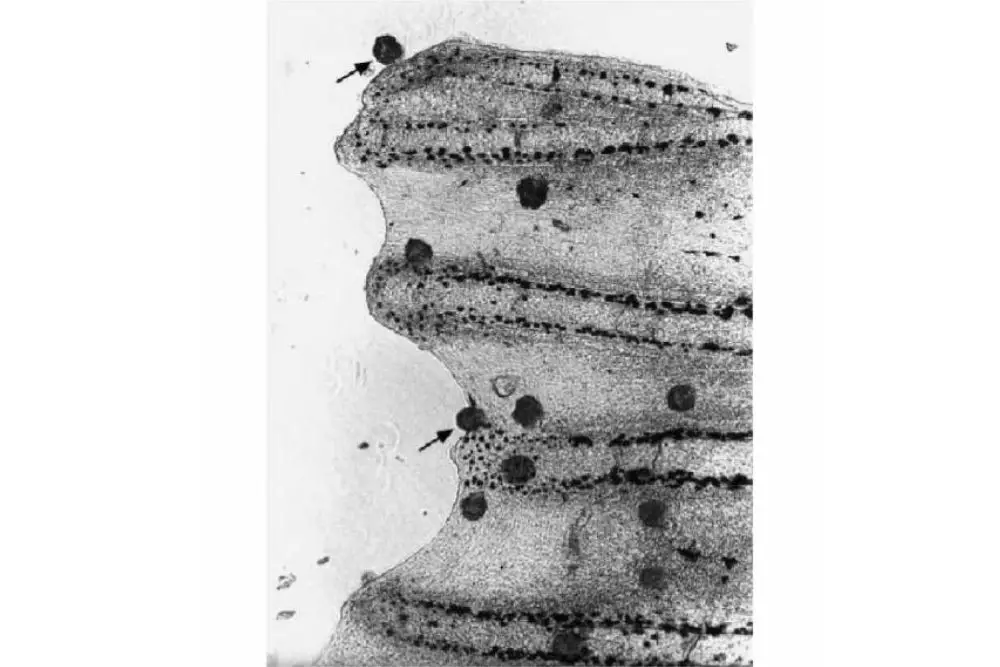
Image Source: Fish Parasites: Pathobiology and Protection book
Sudden change in fish populations and the skin lesions velvety appearance all indicated A. ocellatum infection’s outbreak.
The epithelial hyperplasia, hypertrophy, and lamellar fusions of secondary lamellae of the gills occur.
Gill inflammation were observed histopathological abnormalities in infected fish.

Heavily infestation caused sudden mortality in aquarium, culture unit at high temperature.
Diagnosis of Velvet Disease in Fish
Microscopic examination, histopathological observation and immune diagnosis such as ELISA (Enzyme Linked Immunosorbent Assay) are the main methods of detection the parasite and parasitic infections.
The microscopic findings corroborate the claim that this stage of development (trophonts) was found in skin and gill smears. The parasitic stage (trophonts) was demonstrated through microscopic analysis of gill and skin smears.
Examinations of the gill, fins, and skin mucus (making smear) can be perform to diagnose the presence of desired parasite.
If the parasite is there, it is easily seen using microscopic resolution. Life stage of the parasite that attaches to fish tissue is called the trophont.
The parasite have a pear-shaped to spherical and of dark brown to golden color. Its shape can be seen with very low magnification (40x).
Sequencing and amplification of the small subunit ribosomal RNA gene allowed researchers to identify the parasite as Amyloodinium ocellatum.
Subsequent to polymerase chain reaction amplification and DNA sequencing and phylogenetic analysis, molecular identity of the A. ocellatum isolate was confirmed by means of identification of rDNA fragments by PCR targeting.
Fish Velvet Prevention
Amphiprion frenatus can develop a long-lived and persistent immunological response to the trophont stage of the parasite after repeated nonlethal parasitic attacks.
The protective response is persistent and directed against the specific stage of the parasite.
Fish Velvet Disease Treatment
A test of a combined hyposalination and copper sulfate treatment revealed to have a higher effectiveness than applying copper sulfate alone for alleviating the symptoms of copper sulfate poisoning.
Copper sulphate (0.75 mg l, 12-14 days) baths or freshwater (2-4 minutes) baths are recommended to reduce the trophonts respectively.
The gradual decrease in water salinity as a result of copper sulfate treatment was more effective in preventing the illness than would using copper sulfate alone.
Non-target organisms in the surrounding environment can be affected by copper, malachite green, or methylene blue available as therapies, which has led to these toxic metals being banned from use by the aquaculture industry in several countries.
As a consequence of regulations regarding the use of toxicants, led to the use of the regulated chemicals being prohibited for the aquaculture industry in several regions.
Hydrogen peroxide and peracetic acid are environmentally friendly green antimicrobials that do not leave any residues. Scientists have found hydrogen peroxide to be helpful in treatment of juvenile fish.
Natural feed supplements such as pre- and probiotics can help to improve animal health and minimize stress and infection, respectively.
Freshwater and Marine Velvet Disease
This disease caused by dinoflagellate genera of Amyloodinium and Piscinoodinium is a significant fish health issue affecting cultured freshwater and recreational freshwater fish, and in recent decades has caused a large economic loss to associated industries.
Velvet in Aquarium Fish
In terms of size, fish are one of the biggest classes of pet in the world. There is a growing demand for the veterinary services related to ornamental fish.
Symptoms can be one of the first displays of a large number of infectious and non-infectious diseases, typically diagnosed by the skin.
Goldfish Velvet
Goldfish Velvet is a disease that can affect goldfish. It is caused by a parasitic fungus, and it can cause the fish to develop velvety growths on their skin.
Goldfish Velvet is most commonly found in fish that are kept in dirty tanks or ponds. It is important to keep your goldfish tank clean to prevent the disease from developing.
Goldfish exposed for 21 days to the Oodinium pillularis herb showed a mortality rate of 88%.
Betta Fish Velvet
Betta fish are notorious for getting velvet, a common and easily treatable disease. However, many betta owners don’t know how to spot the early signs of velvet or how to treat it.
Velvet disease is caused by a single-celled protozoan parasite that infects the skin and gills of betta fish.
This parasite feeds on the blood of the fish, causing anemia and lethargy. The infection can also cause damage to the fins and scales, making them appear ragged and discolored.
There are several ways to prevent velvet disease in betta fish. First, make sure to purchase your fish from a reputable dealer who does not keep their bettas in crowded or dirty conditions.
Fish Velvet vs Ich
Though both fish velvet and ich present themselves as white spots on fish, they are two entirely different diseases.
Fish velvet and ich are both parasites that can infect fish. Fish velvet is a type of parasite that can cause whitish appearance on the fish’s skin. Ich is a type of protozoan that can cause white spots on the fish’s skin.
Both of these parasites can be deadly to fish if left untreated.
Fish velvet, also known as marine velvet, is caused by a parasitic dinoflagellate and results in the fish losing its slime coat. Ich, on the other hand, is caused by a ciliated protozoan and results in the fish gasping for air at the surface of the water.
While ich can be treated with a variety of methods, including heat treatment and chemicals, marine velvet is much more difficult to treat.
The most common method of treatment for marine velvet is to remove all affected fish from the tank and treat them in a separate hospital tank.
FAQs
Can humans get velvet from fish?
No, humans cannot get velvet from fish. Although fish do have velvet in their skin, it is not the same type of velvet that is found on deer antlers.
The velvet on fish skin is made up of a different type of protein and is not suitable for human consumption.
References
- Lieke, T., Meinelt, T., Hoseinifar, S. H., Pan, B., Straus, D. L., & Steinberg, C. E. (2020). Sustainable aquaculture requires environmental‐friendly treatment strategies for fish diseases. Reviews in Aquaculture, 12(2), 943-965.
- Sin, Y. M., Ling, K. H., & Lam, T. J. (1992). Protection against velvet disease in goldfish recovered from ichthyophthiriasis. Aquaculture, 102(1-2), 187-191.
- Baticados, M. C. L., & Paclibare, J. O. (1992). The use of chemotherapeutic agents in aquaculture in the Philippines. In Diseases in Asian Aquaculture I. Proceedings of the First Symposium on Diseases in Asian Aquaculture, 26-29 November 1990, Bali, Indonesia (pp. 531-546). Asian Fisheries Society, Fish Health Section.
- Bessat, M., & Fadel, A. (2018). Amyloodiniosis in cultured Dicentrarchus labrax: parasitological and molecular diagnosis, and an improved treatment protocol. Diseases of aquatic organisms, 129(1), 41-51.
- Roberts-Thomson, A., Fielder, D., Barnes, A. C., Lester, R. J., & Adlard, R. D. (2004). Managing velvet disease in marine fish hatcheries.
- Cobb, C. S., Levy, M. G., & Noga, E. J. (1998). Development of immunity by the tomato clownfish Amphiprion frenatus to the dinoflagellate parasite Amyloodinium ocellatum. Journal of Aquatic Animal Health, 10(3), 259-263.
- Francis-Floyd, R., & Floyd, M. R. (2011). Amyloodinium ocellatum, an important parasite of cultured marine fish. Stoneville, MS, USA: Southern Regional Aquaculture Center.
- Walliker, D. (1966). The Management and Diseases of Fish—III Protozoal Diseases of Fish with Special Reference to those Encountered in Aquaria. Journal of Small Animal Practice, 7(12), 799-807.
- Reed, P. A., & Francis-Floyd, R. (1994). Amyloodinium infections of marine fish. Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida.
- Levy, M. G., Litaker, R. W., Goldstein, R. J., Dykstra, M. J., Vandersea, M. W., & Noga, E. J. (2007). Piscinoodinium, a fish-ectoparasitic dinoflagellate, is a member of the class Dinophyceae, subclass Gymnodiniphycidae: convergent evolution with Amyloodinium. Journal of Parasitology, 93(5), 1006-1015.
- SOMMERVILLE, C. (1981). Parasites of ornamental fish. Journal of Small Animal Practice, 22(6), 367-376.
- Pleeging, C. C. F., & Moons, C. P. H. (2017). Potential welfare issues of the Siamese fighting fish (Betta splendens) at the retailer and in the hobbyist aquarium. Vlaams Diergeneeskundig Tijdschrift, 86(4).
- Murphy, K. M., & Lewbart, G. A. (1995, October). Aquarium fish dermatologic diseases. In Seminars in Avian and Exotic pet medicine (Vol. 4, No. 4, pp. 220-233). WB Saunders.
- Rao, M. V., Kumar, T. A., & Haq, M. B. (2013). Diseases in the Aquarium fishes: Challenges and areas of concern: An overview. International journal of environment, 2(1), 127-146.
- Noga, E. J. (2012). Amyloodinium ocellatum. Fish Parasites: Pathobiology and Protection; CABI: Wallingford, UK, 19-29.
- Ramesh Kumar, P., Nazar, A. A., Jayakumar, R., Tamilmani, G., Sakthivel, M., Kalidas, C., & Gopakumar, G. (2015). Amyloodinium ocellatum infestation in the broodstock of silver pompano Trachinotus blochii (Lacepede, 1801) and its therapeutic control. Indian Journal of Fisheries, 62(1), 131-134.
- Cascarano, M. C., Stavrakidis-Zachou, O., Mladineo, I., Thompson, K. D., Papandroulakis, N., & Katharios, P. (2021). Mediterranean aquaculture in a changing climate: temperature effects on pathogens and diseases of three farmed fish species. Pathogens, 10(9), 1205.
- Ferraz, E., & Sommerville, C. H. R. I. S. T. I. N. A. (1998). Pathology of Piscinoodinium sp.(Protozoa: Dinoflagellida), parasites of the ornamental freshwater catfishes Corydoras spp. and Brochis splendens (Pisces: Callichthyidae). Diseases of Aquatic Organisms, 33(1), 43-49.
- Saraiva, A., Jeronimo, D., & Cruz, C. (2011). Amyloodinium ocellatum (Chromalveolata: Dinoflagellata) in farmed turbot. Aquaculture, 320(1-2), 34-36.
- Alvarez-Pellitero, P. (2004). Report about fish parasitic diseases. Etudes et Recherches, Options Mediterranennes. CIHEAM/FAO, Zaragoza, 103-130.
- Monvises, A., Nuangsaeng, B., Sriwattanarothai, N., & Panijpan, B. (2009). The Siamese fighting fish: well-known generally but little-known scientifically. ScienceAsia, 35(1), 8-16.
- Marques, C. L., Medeiros, A., Moreira, M., Quental-Ferreira, H., Mendes, A. C., Pousao-Ferreira, P., & Soares, F. (2019). Report and genetic identification of Amyloodinium ocellatum in a sea bass (Dicentrarchus labrax) broodstock in Portugal. Aquaculture Reports, 14, 100191.
- Lima, A. F., Rodrigues, A. P. O., Oliveira-Maciel, P., Prysthon, A., Valladao-Flores, R. M., & Araujo-Bezerra, T. (2018). Small-scale fish farming in seasonal ponds in Brazil: technical and economic characterization. Latin american journal of aquatic research, 46(2), 314-329.
- Martins, M. L., Cardoso, L., Marchiori, N., & Benites de Pádua, S. (2015). Protozoan infections in farmed fish from Brazil: diagnosis and pathogenesis. Revista Brasileira de Parasitologia Veterinaria, 24, 1-20.
- Cecchini, S., Saroglia, M., Terova, G. and Albanesi, F. (2001). Detection of antibody response against Amyloodinium ocellatum (Brown, 1931) in serum of naturally infected European sea bass by an enzyme-linked immunosorbent assay (ELISA). Bull. Eur. Ass. Fish Pathol., 21(3): 104-108.
- Cobb, C.S., Levy, M.G. and Noga, E.J. (1998). Acquired immunity to amyloodiniosis is associated with an antibody response. Dis. Aquat. Org., 34: 125-133.
- Montgomery-Brock, D., Sato, V.T., Brock, J.A. and Tamaru, C.S. (2001). The application of hydrogen peroxide as a treatment for the ectoparasite Amyloodinium ocellatum (Brown 1931) on the Pacific threadfin Polydactylus sexfilis. J. World Aquac. Soc., 32: 250-254.
- Paperna, I. (1980). Amyloodinium ocellatum (Brown, 1931) (Dinoflagellida) infestations in cultured marine fish at Eilat, Red Sea: Epizootiology and pathology. J. Fish Dis., 3: 363-372.