Ich is a common parasitic infection caused by a protozoan parasite that affects the skin and gills of fish, resulting in the appearance of white spots or lesions on their bodies.
In this article, we will explore the causes and contributing factors of ich, common symptoms of the infection, and various treatment options available to help combat the disease.

What is Ich and What Causes Ich on Fish
Ich is the short form of Ichthyophthirius multifiliis, a protozoan (Eukaryote, unicellular) parasite member of the family Ichthyophthiriidae.
The name “Ich or Ick” comes from the disease causing parasite’s (Ichthyophthirius multifiliis) first three letters.
The scientific names comes from Greek words ichthys = Fisch, phtheiros = louse; Latin: multus = much, filia = daughter.
In a normal eye view, it looks like white spots on the body, eye, gill and fins of the fish. The spots on fish may be small or large.
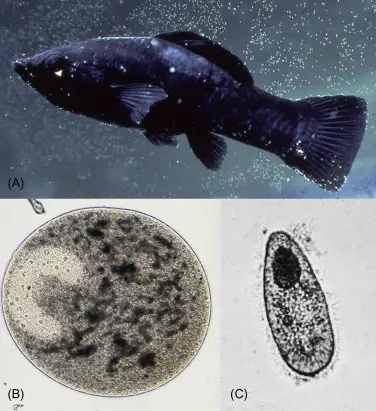
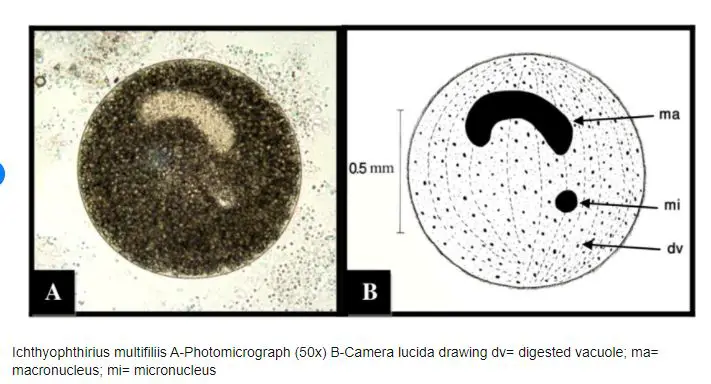
In microscope view, it looks circular shaped and has cilia surrounding its body. It has a horseshoe-shaped macronucleus that is typical to it and an identifying feature.
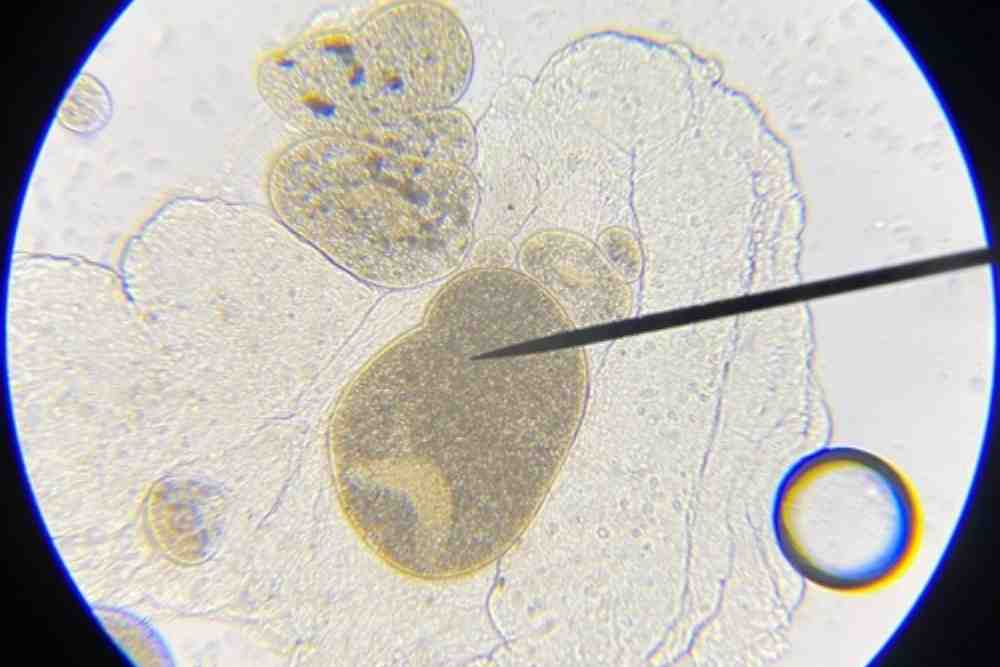
It is a free-swimming ciliated protozoan, has cilia that cover the body completely. The pear-shaped infective ciliated theronts are usually motile and active, approximately 30 to 45 micrometres long.
Ich larvae enter the fish’s body through the gills and skin and then migrate to the muscles and internal organs where they mature and reproduce.
Ichthyophthirius multifiliis causes circular white spots that appear on the skin and gill of infected fish where it feeds on the fish’s blood and tissue.
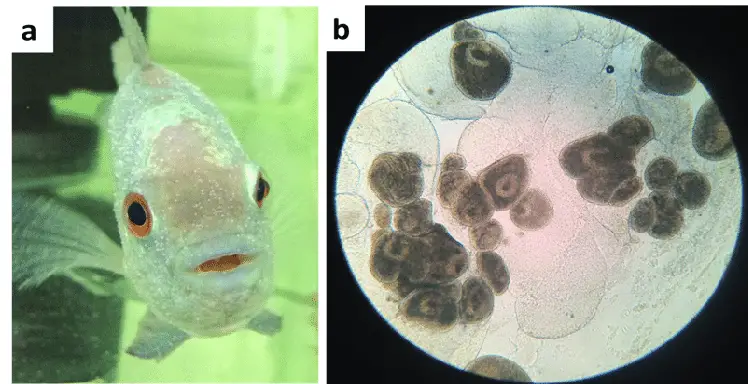
The white spot on fish is a circular, white lesion that is found on the skin of fish. This small, round spot is located on the fish’s dorsal fin and is usually white or light-colored.
The ick can cause a number of direct and indirect (secondary) problems for the fish, including inflammation, lesions, fungal, bacterial infection and physical injury.
Morphology of Ich (Ichthyophthirius multifiliis)
Ichthyophthirius multifiliis is a parasitic ciliate that infects freshwater fish. Its morphology can be described as follows:
- Size: Ich is a relatively large ciliate, with a body length ranging from 200 to 1000 micrometers (µm).
- Shape: Ich has a rounded, flattened shape and is covered in cilia, which it uses for locomotion and feeding.
- Suction cups: The body of Ich is covered in numerous small suction cups, called tomonts, which it uses to attach to the skin of fish.
- Nucleus: Ich has a large, horseshoe-shaped nucleus that is visible under a microscope. This nucleus contains the genetic material of the parasite.
- Vacuoles: Ich has several vacuoles within its body, which it uses to store food and waste products.
- Reproduction: Ich has a complex life cycle that involves both sexual and asexual reproduction. During the asexual phase, Ich multiplies rapidly and produces hundreds of daughter cells, which eventually burst out of the tomonts and infect new fish. During the sexual phase, two Ich cells fuse together to form a cyst, which then divides into numerous daughter cells.
- Cytostome: Ich has a cytostome, or a mouth-like opening, which it uses to ingest food particles. The cytostome is located on the ventral side of the parasite.
- Contractile vacuole: Ich has a contractile vacuole, which it uses to regulate its water balance. The contractile vacuole is located near the posterior end of the parasite and pumps out excess water that enters the cell.
- Macronucleus: In addition to the horseshoe-shaped nucleus, Ich also has a large, elongated macronucleus that is involved in gene expression and protein synthesis.
- Sarcoplasmic reticulum: Ich has a well-developed sarcoplasmic reticulum, which is a network of tubules and vesicles that plays a role in muscle contraction. This allows the parasite to move and feed efficiently.
- Adhesive disc: Ich has an adhesive disc located on its ventral side, which it uses to attach to the skin of fish. The adhesive disc is composed of specialized cilia that secrete a sticky substance.
- Cilia: Ich is covered in numerous cilia, which it uses for locomotion and feeding. The cilia are arranged in rows along the surface of the parasite, creating a rippling motion that propels it forward.
Fish Species Affected by Ichthyophthirius multifiliis
Freshwater fish
- Rui
- Catla
- Mrigal
- Common Carp
Marine fish
- Clownfish
- Tangs and
- Damselfish
Game fish
- Bass,
- Trout and
- Salmon
Ornamental fish
- Goldfish
- Angelfish
- Cichlids
- Tetras and
- Guppies
Geographical Distribution of Ich (Ichthyophthirius multifiliis)
Ich has been reported in fish from all continents, and it is likely present in most countries with aquatic ecosystems.
It is particularly common in tropical and subtropical regions, where the warm water temperatures and high humidity favor the proliferation of the parasites.
Ich outbreaks can occur in aquaculture facilities, public aquariums, and home aquariums, as well as in natural bodies of water.
Ich usually found in freshwater captive fish species and they are very specific to host species and have very low host specificity.
Transmission of Ich (Ichthyophthirius multifiliis)
Ich parasites can be transmitted from one water body to another or one aquarium to another through several means. Some common ways that ich parasites can spread include:
- Movement of infected fish: If infected fish are introduced into a new water body or aquarium, they can transmit the parasites to the other fish in the system.
- Transfer of contaminated water: Ich parasites can survive for a short period of time in the water, so if contaminated water is introduced into a new system, the parasites can spread to the fish in the new system.
- Use of contaminated equipment: If contaminated equipment, such as nets, buckets, or filters, is used in multiple systems, it can spread the parasites to the new system.
- Transfer of contaminated plant material: Ich parasites can also be transmitted through the transfer of contaminated plant material, such as live plants or decorations.
- Transfer of contaminated substrates: Contaminated substrates like, sand, gravel and driftwood carry the parasite.
Life Cycle of Ich (Ichthyophthirius multifiliis)
The life cycle of ich consists of three stages: trophont, tomont and theront.
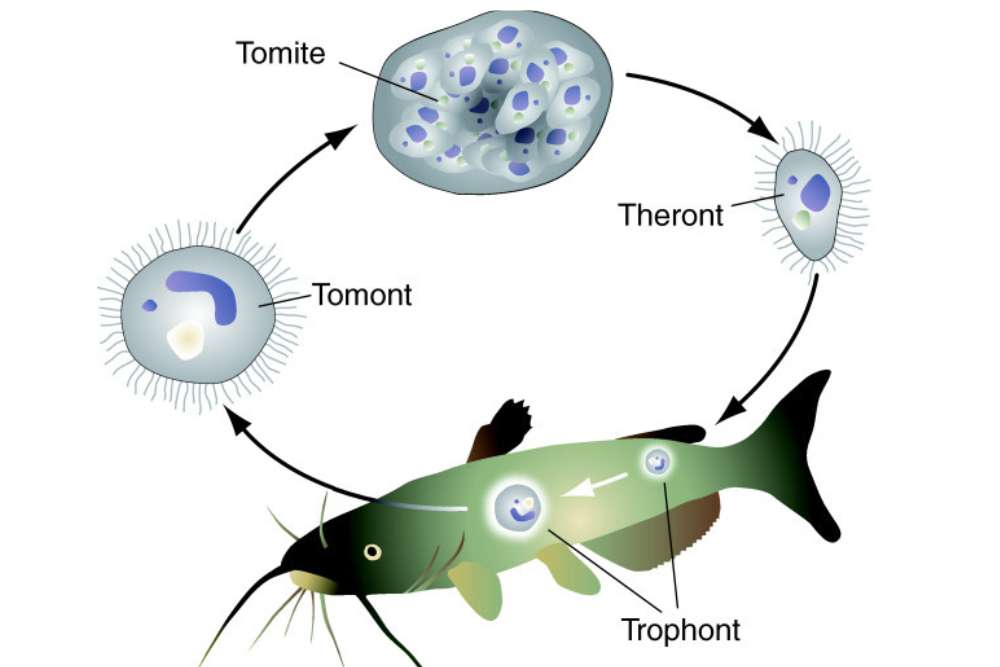
1. Trophont
This is the infective stage of the parasite. The trophont is a stationary, feeding stage that is attached to the skin or gills of the fish.
During this stage, the trophont feeds on the blood cells, host’s tissues (cellular debris and live host cells) and secretes a protective cyst around itself.
This stage have a rounded anterior end and a posterior end with a distinct constriction. It contain a single nucleated cell and a prominent nucleus. It lack cilia but have a thin layer of cilia on the outer edge of the body.
Fish infected by trophonts stage become very sick. It causes fish to lose weight and eventually die.
2. Tomont
When the trophont has matured, stops feeding at its environment and detach from the host (fish) and turn into a tomont. A mature trophont lacks the energy to reproduce.
In water, the tomont immediately produces a tomitical cyst that has a tough, gelatinous wall. When the individual encapsulated in cyst it prepare itself for next generation.
Inside the cyst, within days the single tomont cell divide multiple times and produce hundred to thousands of infective, ciliated tomites at a water temperature of 22° to 25ºC (72º to 77ºF).
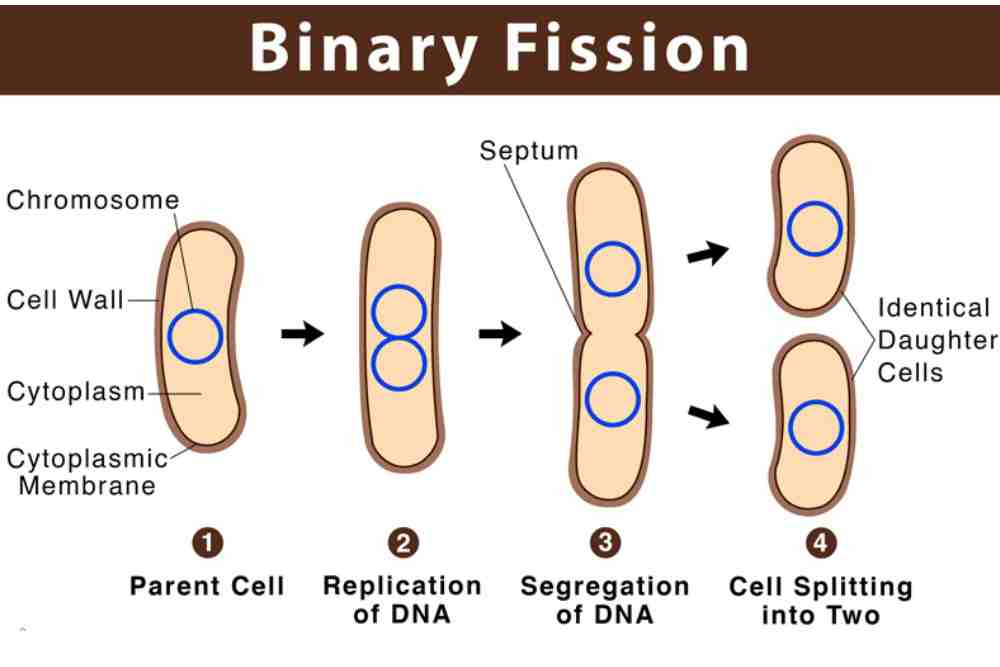
It’s important to note that Ich can also reproduce asexually, through a process called binary fission. This allows the parasite to rapidly increase its population size, which can lead to outbreaks of disease in fish populations.
3. Theront
Theronts begin to develop within the tomont cyst and develop into larval theront within a tomont. Theront development within these cysts occurs over 1 (warm water temperature) to 3 week period (cold water temperature).
The released or hatched larval form is called a theront. The theront is a swimming, motile, nonfeeding stage of the parasite.
The theronts are pear shaped and range in size from 0.1 to 0.3 millimeter in diameter. These young are transparent and move rapidly along the length of the body.
The released theronts desperately swim in the water 2-3 days for their next host.
Interesting fact is, if they do not manage to find their next host within this short time frame, they will die and their life cycle will not be fulfilled.
However, once they find their host immediately they attach on their skin and burrow onto its epithelial tissue (outer covering of the skin).
The epithelial tissue covers the entire body’s surface, and it guards the body from mechanical injury, chemical exposure, excessive fluid loss, and infection.
When the dig into the fish epithelium, the theront will eventually develop into a trophont and the cycle will start again.
What Causes of Ich on Fish?
The causative agent of white spot disease is Ichthyophthirius multifiliis. The parasite can be introduced into a fish tank through new fish, untreated water, or food.
There are several factors that can contribute to the presence of ich in an aquarium and commercial water body. These factors are,
- Poor Water Quality: Poorly maintained tanks with low or high pH, high levels of ammonia and nitrite can weaken fish and make them more susceptible to ich.
- Stress: Fish that are stressed due to overcrowding, poor nutrition, or other factors may be more likely to develop ich.
- Temperature: Ich tends to thrive in cold water, so keeping the temperature of the tank too low can increase the risk of an outbreak.
- Poor Tank Hygiene: Dirty tanks and equipment can harbor ich parasites and lead to an outbreak. Net and hoses may transfer the parasite one tank to another.
- Inadequate Quarantine: Introducing new fish or fry to the tank without properly quarantining them can allow ich parasites to enter the tank.
- Overcrowded Fish: The illness is comparatively severe when fish are overcrowded.
- Immunity: Some fish may have a natural immunity to ich, while others may be more susceptible.
Signs & Symptoms of Ich on Fish
White spots: The most common symptom of Ich is the appearance of small, white spots on the fish’s body, fins, and gills. These spots may look like grains of salt or sugar and can be easily seen on the fish.

These spots are actually the parasites, which can range in size from very small to visible to the naked eye. The spots may grow and spread until large areas of the body are covered.
- Scratching and rubbing: Infected fish may scratch or rub against objects in the tank or pond, as the parasites cause irritation and discomfort.
- Lethargy: Fish infected with Ich may appear lethargic or inactive, and may not feed as usual.
- Respiratory distress: In severe cases of Ich, fish may show signs of respiratory distress, such as gasping at the surface or rapid breathing.
- Behavioral changes: Ich can cause behavioral changes in fish, such as hiding or schooling differently than usual.
- Loss of appetite: Fish infected with Ich may lose their appetite or eat less than usual due to the discomfort caused by the parasites.
- Cloudy eyes: Some fish with Ich may develop cloudy or hazy eyes, which is a sign of an advanced infection.
- Flicking and flashing: Infected fish may flick their fins or flash their bodies in an attempt to dislodge the parasites.
- Stress: Ich can cause stress in fish, which can make them more susceptible to other infections and diseases.
- Weakened immune system: Prolonged infections with Ich can weaken the fish’s immune system, making them more vulnerable to other infections and diseases.
Diagnosis of Ich (Ichthyophthirius multifiliis)
The diagnosis of Ichthyophthiriasis is typically made based on the clinical signs and symptoms that are observed in infected fish.
However, it is important to note that the clinical signs of Ichthyophthiriasis can vary depending on the stage of the disease, the severity of the infection, and the species of fish that is infected.
According to a study published in the Journal of Aquatic Animal Health, the clinical signs of Ichthyophthiriasis can include the presence of white, grain-like spots on the skin and fins of infected fish, as well as increased mucus production, respiratory distress, and behavioral changes such as lethargy and loss of appetite.
To confirm a diagnosis of Ichthyophthiriasis, veterinarians and fish health professionals may use various diagnostic techniques, including microscopic examination of skin scrapings or gill tissue, histopathology, and PCR-based molecular methods.
Microscopic examination of skin scrapings or gill tissue is one of the most commonly used diagnostic methods for Ichthyophthiriasis.
In this technique, a small sample of skin or gill tissue is collected from the infected fish and examined under a microscope to look for the presence of Ichthyophthirius multifiliis.
This method can be effective in detecting the parasite in the early stages of infection, but it may not be reliable in cases where the parasite is present in low numbers.
Histopathology is another diagnostic technique that can be used to confirm a diagnosis of Ichthyophthiriasis.
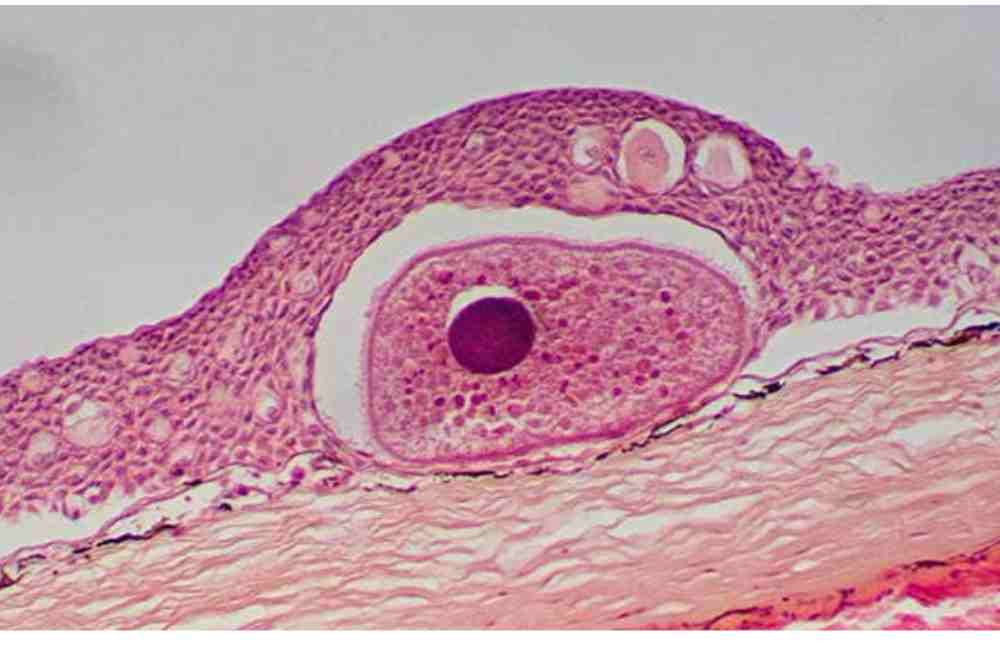
In this technique, a tissue sample is collected from the infected fish and examined under a microscope to look for the presence of the parasite and any associated tissue damage or inflammation.
This method can provide more detailed information about the extent of the infection and the severity of the tissue damage, but it can be more time-consuming and expensive than other diagnostic methods.
PCR-based molecular methods are a newer and more advanced diagnostic technique that can be used to detect the presence of Ichthyophthirius multifiliis in fish tissues.
How To Prevent Ich on Fish
Prevention of ich is the best course of action, and there are several things that can be done to reduce the risk of a fish becoming infected.
By following these steps, you can help to prevent ich and other diseases from affecting your aquarium fish.
Separate Infected Fish
Remove affected fish from the tank and treat them in a separate container with medication. The water in the main tank should then be treated with similar medication.
It is also important to clean all of the equipment in the tank and replace the water with fresh water. The quarantine period helps prevent the spreading of the disease.
Quarantine New Fish
Quarantine new fish for at least two weeks before introducing them to your main tank.
This will allow you to monitor them for any signs of disease, including ich, and treat them if necessary before they have a chance to infect your other fish.
Avoid introducing wild fish or plants to your aquarium, as these can be carriers of diseases such as ich.
Maintain Good Water Quality | Keep Aquarium Clean
The most important step is to maintain good water quality if you notice ich. A clean, healthy aquarium is less likely to be affected by ich and other diseases.
Keep your aquarium clean and well-maintained by performing regular water changes, keeping the tank free of excess food and debris and maintaining the proper pH and temperature.
Make sure your tank or water is properly cycled and filtered, and keep the water temperature within the appropriate range.
Avoid Overstocking
Avoid overstocking your tank, as overcrowding can lead to increased stress and a higher risk of disease.
Provide Provide Nutritional Diet
Another important step is to provide your fish with a healthy diet. Feed your fish a varied diet of high-quality, nutritious foods to help boost their immune system and make them less prone to illness.
If you have an ich outbreak in your tank, be sure to treat all of your fish, even if they don’t appear to be affected.
Ich is highly contagious and can spread quickly through an aquarium, so it’s important to treat all of your fish to ensure that the disease is fully eradicated.
Ich on Fish Treatment
It’s advisable to treat infectious theronts and reproductive tomonts when they’re still in the water and before they have the opportunity to penetrate the fish as trophonts.
Free swimming infective theronts are susceptible to chemical treatment. Chemical treatment have to done to control free-swimming theronts by applying chemicals.
1. Increase Water Temperature
Water temperature is a crucial factor for the control of these parasitic worms. Warm water helps kill Ich.
Raising the water temperature several degrees Celsius above normal raising the water temperature for 5 to 7 days hampers the growth of pathogens that are sensitive to heat and boosts the immune response of the host.
2. Chemical Treatment
To a significant extent, all anti-ich chemical substances have formaldehyde, malachite green, copper sulfate, or a combination of one or more of formaldehyde, malachite green, copper sulfate, and malachite green.
Formaldehyde at 0.25 ppm (1 ml/10 gallons) is effective if administered three times on alternating days.
Formalin is also used as an effective treatment against this parasitic disease. But in the case of low dissolved oxygen levels, formalin is not suggested. 0.1 ppm malachite green (zinc-free) is used for treating sick fish.
3. Formalin Bath
Formalin is a common treatment for Ichthyophthiriasis. According to Szekely and Molnar (2019), a 10-15 minute bath in a solution of formalin at a concentration of 250-500 ppm can be effective in killing Ichthyophthirius multifiliis on the fish.
However, formalin can be toxic to fish at high concentrations, so it is important to follow the recommended dosage carefully and monitor the fish during treatment.
4. Copper Sulfate (CuSO4)
Copper sulfate is another commonly used treatment for Ichthyophthiriasis. According to Rose et al. (1979), a 30-60 minute bath in a solution of copper sulfate at a concentration of 0.2-0.3 ppm can be effective in killing Ichthyophthirius multifiliis on the fish.
However, copper sulfate can also be toxic to fish at high concentrations, so it is important to follow the recommended dosage carefully and monitor the fish during treatment.
5. Malachite Green
Malachite green is a dye that has been used for many years to treat Ichthyophthiriasis. According to Szekely and Molnár (2019), a 30-60 minute bath in a solution of malachite green at a concentration of 0.1-0.5 ppm can be effective in killing Ichthyophthirius multifiliis on the fish.
However, malachite green is carcinogenic and can be toxic to fish at high concentrations, so it is important to follow the recommended dosage carefully and use it only as a last resort.
6. Salt bath
A bath in a salt solution can help to reduce the severity of Ichthyophthiriasis. According to Szekely and Molnar (2019), a 30-60 minute bath in a solution of aquarium salt at a concentration of 2-3% can help to reduce the number of parasites on the fish. However, this treatment may not completely eliminate the parasites and should be used in combination with other treatments.
7. Combination Treatments
Combination treatments using two or more medications can be more effective in treating Ichthyophthiriasis than using a single medication alone.
According to Szekely and Molnar (2019), a combination of formalin and malachite green, or formalin and copper sulfate, can be effective in treating Ichthyophthirius multifiliis in fish.
Treatment of Ichthyophthiriasis in Rainbow Trout and Common Carp
Rainbow trout are typically treated with five repeated treatments of formalin at 110 lL/L for 1 hour, or with Aquahumin at 100-150 lL/L for 2 hours, with treatments administered at 24-hour intervals.
At a temperature of 18-20°C, five treatments at 24-hour intervals with formalin at 110 lL/L can kill all parasites in common carp.
In vivo experiments have shown that three treatments (at 24-hour intervals) with formalin at 110 lL/L or Aquahumin at 150 lL/L, or a single long-term treatment with formalin at 110 lL/L for 12 hours, can be effective in eliminating all parasites in rainbow trout.
Common carp, on the other hand, require five treatments of formalin at 110 lL/L for 2 hours to completely remove all parasites. Additionally, Aquahumin has been found to be effective in completely eliminating I. multifiliis infestations in common carp but is less effective than formalin.
Efficacy of quinine against ichthyophthiriasis in common carp (Cyprinus carpio). In vitro experiments indicated that higher concentrations of quinine were more effective at killing the parasite, with the majority of trophonts dead at the highest concentration. Theronts were highly sensitive to the treatment, while tomonts were less affected.
In the in vivo experiments, injections of quinine resulted in a reduction in the number of parasites in the fish, but there was no significant difference in the number of trophonts between the quinine-treated groups and the control group in the in-feed trials. Quinine administered through feed was well-tolerated by the fish, but uptake diminished at higher concentrations.
8. Regular Water Exchange
The most important step is to improve water quality by doing regular water changes and add filtration system in water body.
You can also help reduce stress on your fish by providing a stress-free environment and avoiding sudden changes in temperature. Perform a partial water change and clean the filter to remove any excess medication and parasites from the tank.
Continue to monitor your fish closely and treat for an additional 3-5 days after the visible signs of ich have disappeared to ensure that all of the parasites have been eliminated.
White Spot But Not Caused By Ich
White spots on fish can be caused by a variety of things, including parasites, fungal infections, and physical injuries.
If the white spots are not caused by ich (also known as white spot disease), it is important to determine the underlying cause in order to properly treat the condition.
Some possible causes of white spots on fish include:
- Velvet disease: A parasite that causes small, gold or brown spots on the skin of fish.
- Columnaris: A bacterial infection that causes white patches or lesions on the skin or fins of fish.
- Fungal infections: Fungal infections can cause white or gray patches on the skin or fins of fish.
- Physical injuries: Physical injuries, such as cuts or abrasions, can also cause white spots on fish.
Frequently Asked Questions (FAQs)
Can white spot affect humans?
Ich parasites do not infect humans and cannot be transmitted to humans from fish.
However, humans can be infected with other parasites that may be transmitted through fish, such as tapeworms and certain types of flukes. These parasites typically require intermediate hosts, such as snails or crustaceans, to complete their life cycle.
Humans can become infected if they eat raw or undercooked fish that is contaminated with these parasites. To reduce the risk of infection, it is important to cook fish thoroughly and to practice good hygiene when handling raw fish.
Can white spot kill fish?
Ich can be a serious and potentially fatal disease for fish. The parasite interfere with the fish’s ability to breathe and regulate its body temperature, which can weaken the fish and make it more susceptible to other diseases.
In severe cases, ich can lead to significant morbidity and mortality in fish. The mortality rate may vary depending on the species of fish, the level of infection, and the overall health of the fish. Some fish, particularly those that are stressed or immunocompromised, may be more vulnerable to the effects of ich.
How long does ich stay on fish?
The ich life cycle is relatively short, lasting only about 10 days, but during that time the parasite can infect and kill vast numbers of fish.
References
- Matthews, R. A. (2005). Ichthyophthirius multifiliis Fouquet and ichthyophthiriosis in freshwater teleosts. Advances in parasitology, 59, 159-241.
- Cross, M. L., & Matthews, R. A. (1993). Ichthyophthirius multifiliis Fouquet (Ciliophora): the location of sites immunogenic to the host Cyprinus carpio (L.). Fish & Shellfish Immunology, 3(1), 13-24.
- Ewing, M. S., Kocan, K. M., & Ewing, S. A. (1983). Ichthyophthirius multifiliis: morphology of the cyst wall. Transactions of the American Microscopical Society, 122-128.
- Wei, J. Z., Li, H., & Yu, H. (2013). Ichthyophthiriasis: emphases on the epizootiology. Letters in Applied Microbiology, 57(2), 91-101.
- Huang, K., Hu, G., Wang, R., Zeng, Q., Li, W., Zou, H., … & Li, M. (2022). In Vitro Assessment of Berberine against Ichthyophthirius multifiliis in Goldfish. Pathogens, 11(10), 1207.
- Chandra, K. J. (2008). A practical text book of fish Parasitology and health management. The Bangladesh University Grants Commission, Agargaon, Sher-e-Bangla Nagar, Dhaka, Bangladesh. 213p.
- Witeska, M., Kondera, E., & Lugowska, K. (2010). The effects of ichthyophthiriasis on some hematological parameters in common carp. Turkish Journal of Veterinary & Animal Sciences, 34(3), 267-271.
- von Gersdorff Jørgensen, L. (2016). Infection and immunity against Ichthyophthirius multifiliis in zebrafish (Danio rerio). Fish & Shellfish Immunology, 57, 335-339.
- Olsen, M. M., Kania, P. W., Heinecke, R. D., Skjoedt, K., Rasmussen, K. J., & Buchmann, K. (2011). Cellular and humoral factors involved in the response of rainbow trout gills to Ichthyophthirius multifiliis infections: molecular and immunohistochemical studies. Fish & shellfish immunology, 30(3), 859-869.
- Gonzalez, S. F., Buchmann, K., & Nielsen, M. E. (2007). Real-time gene expression analysis in carp (Cyprinus carpio L.) skin: inflammatory responses caused by the ectoparasite Ichthyophthirius multifiliis. Fish & shellfish immunology, 22(6), 641-650.
- Gonzalez, S. F., Buchmann, K., & Nielsen, M. E. (2007). Ichthyophthirius multifiliis infection induces massive up-regulation of serum amyloid A in carp (Cyprinus carpio). Veterinary immunology and immunopathology, 115(1-2), 172-178.
- Buchmann, K., Sigh, J., Nielsen, C. V., & Dalgaard, M. (2001). Host responses against the fish parasitizing ciliate Ichthyophthirius multifiliis. Veterinary parasitology, 100(1-2), 105-116.
- Wei, J. Z., Li, H., & Yu, H. (2013). Ichthyophthiriasis: emphases on the epizootiology. Letters in applied microbiology, 57(2), 91–101.
7 thoughts on “Ich on Fish: Causes, Symptoms and Treatment”
Comments are closed.