Marine velvet, caused by the dinoflagellate parasite Amyloodinium ocellatum, is a parasitic disease that affects fish in marine and freshwater environments. It is highly contagious and can impact a wide range of fish species, causing various symptoms such as a yellow or gold dust-like appearance on the skin and gills, lethargy, loss of appetite, and respiratory distress.
Understanding the causes, symptoms, and treatment options for marine velvet is crucial for fish keepers and aquaculture practitioners in order to effectively manage and prevent outbreaks of this disease.
In this article, we will provide an overview of marine velvet, including its causes, symptoms, and treatment strategies, to help raise awareness about this parasitic disease and promote proper management practices for fish health.
What is Marine Velvet? (Amyloodinium ocellatum)
Marine velvet, also known as marine velvet disease or simply “velvet,” is a parasitic disease that affects fish in marine and freshwater environments. It is caused by a dinoflagellate parasite called Amyloodinium ocellatum.
Marine velvet is highly contagious and can infect a wide range of fish species, including both coldwater and tropical fish.
Causes of Marine Velvet Disease | Red Velvet Disease
Marine velvet is caused by a parasitic dinoflagellate known as Amyloodinium ocellatum. This microscopic organism is a type of plankton that can infect fish in marine and freshwater environments.
Poor water quality, stress, overcrowding, and other environmental factors can weaken fish and make them more susceptible to infection by A. ocellatum.
Morphology of Amyloodinium ocellatum
Amyloodinium ocellatum is a dinoflagellate parasite that causes marine velvet in fish. It has some distinct physical characteristics:
Size: A. ocellatum is a microscopic organism, with an average size of about 20-250 micrometers, depending on the life stage. Dinospores, which are the infective stage, are smaller, typically ranging from 20-30 micrometers, while trophonts, the feeding stage, can be larger, ranging from 30-250 micrometers.
Shape: A. ocellatum has a rounded or oval shape, with a characteristic dinoflagellate cell structure. It has a distinct cell membrane and a flagellum that it uses for movement.
Color: A. ocellatum is typically colorless or translucent in its dinospore and trophont stages, making it difficult to see with the naked eye. However, trophonts can appear yellow or gold dust-like when attached to fish, which gives the infected fish a velvety or shimmering appearance.
Cell wall: A. ocellatum has a complex cell wall structure, which includes a cellulose plate or theca that encases the cell. This theca can have distinct patterns and shapes, which can be useful in identifying the species under a microscope.
Cyst: A. ocellatum can also form cysts, known as tomonts, as part of its life cycle. Tomonts are encysted stages that fall to the bottom of the aquarium or fish farm and can be round or oval in shape, with a protective outer covering.
Amyloodinium ocellatum Life Cycle
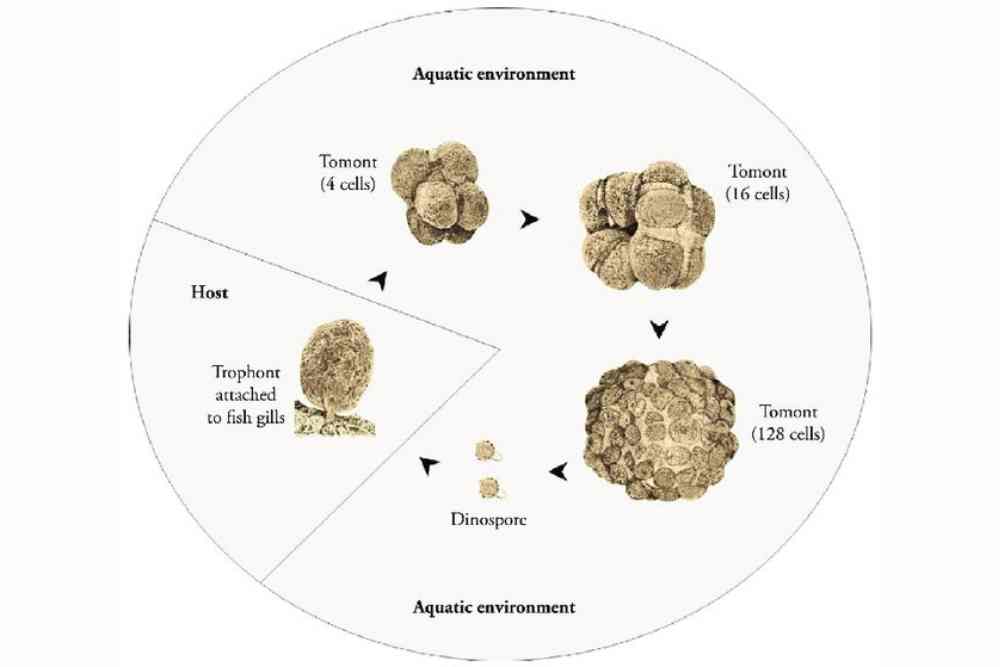
Amyloodinium ocellatum is a parasitic dinoflagellate that causes marine velvet disease in fish. The life cycle of A. ocellatum involves several stages, including:
Dinospores: The life cycle of A. ocellatum begins with the release of dinospores from the mature trophont, which is the parasitic stage of the organism. Dinospores are free-swimming and infectious, and they can attach to fish hosts using specialized structures called tentacles.
Trophonts: Once dinospores attach to a fish host, they transform into trophonts, which are the feeding stage of A. ocellatum. Trophonts embed themselves in the skin and gills of the host fish, where they feed on the host’s cells and blood. Trophonts can reproduce asexually by dividing into multiple daughter cells, which then develop into new trophonts.
Tomonts: After feeding and maturing, the trophonts transform into tomonts, which are the reproductive stage of A. ocellatum. Tomonts are typically enclosed in a protective cyst, and they can detach from the fish host and settle in the environment, such as on the substrate or equipment in the aquarium. Inside the cyst, tomonts undergo multiple rounds of cell division to produce numerous daughter cells called tomites.
Tomites: Tomites are the infective stage of A. ocellatum. They are released from the tomont cysts and are capable of swimming and seeking out new fish hosts to attach to. Once attached to a fish host, tomites transform into dinospores, and the life cycle of A. ocellatum begins again.
The life cycle of A. ocellatum can be completed within a few days, and the parasitic dinoflagellate can rapidly reproduce and spread in fish populations, causing significant damage to fish health and aquaculture operations.
Marine Velvet Symptoms
The symptoms of Marine Velvet Disease, caused by Amyloodinium ocellatum, can vary depending on the stage of the infection and the severity of the disease. Here are some common symptoms associated with Marine Velvet Disease:
Yellow or gold dust-like appearance on the skin and gills: One of the hallmark signs of Marine Velvet Disease is the presence of tiny yellow or gold flecks or dust-like particles on the skin, fins, and gills of affected fish. These particles are actually the dinospores of A. ocellatum, which are the mobile stage of the parasite.
Lethargy and loss of appetite: Fish infected with Marine Velvet Disease may exhibit lethargy, reduced activity levels, and loss of appetite. They may appear sluggish or show a decreased interest in food, which can lead to weight loss and a decline in overall health.
Respiratory distress: Infected fish may exhibit signs of respiratory distress, such as rapid or labored breathing, increased opercular movement (gill flaring), or gasping at the water surface. This can be a result of gill damage caused by the parasite, leading to compromised respiratory function.
Behavioral changes: Marine Velvet Disease can also cause behavioral changes in fish, such as increased hiding, clamped fins, rubbing against surfaces, or flashing (sudden erratic movements) in an attempt to alleviate the discomfort caused by the parasite.
Skin and fin erosion: In severe cases, Marine Velvet Disease can cause skin and fin erosion or ulceration in fish. The skin may appear ragged or frayed, and fins may show signs of damage or decay.
Stress-related symptoms: The stress caused by the presence of A. ocellatum and the resulting disease can weaken the immune system of infected fish, making them more susceptible to secondary infections by other pathogens. This can lead to additional symptoms such as increased susceptibility to other diseases, fin rot, or development of secondary bacterial or fungal infections.
Diagnose of Marine Velvet Disease
Diagnosing Marine Velvet Disease, caused by Amyloodinium ocellatum, typically involves a combination of clinical signs, microscopic examination, and water quality analysis. Here are some common methods used for diagnosis:
Clinical signs: Observation of characteristic symptoms in affected fish can provide important clues for diagnosing Marine Velvet Disease. These may include yellow or gold dust-like appearance on the skin and gills, lethargy, loss of appetite, respiratory distress, and behavioral changes. However, these symptoms can also be indicative of other diseases, so further diagnostic methods are needed for confirmation.
Microscopic examination: Microscopic examination of fish skin and gill tissue can help confirm the presence of A. ocellatum. Skin or gill scrapes are taken from affected fish and examined under a microscope for the presence of dinospores, trophonts, or tomonts of A. ocellatum. These microscopic stages of the parasite are distinctive in size, shape, and cellular structure, and can be identified by an experienced professional.
Water quality analysis: Poor water quality, including high ammonia, nitrite, or nitrate levels, and low oxygen levels, can stress fish and make them more susceptible to Marine Velvet Disease. Analyzing water quality parameters can help identify any environmental factors that may have contributed to the outbreak of the disease.
Polymerase Chain Reaction (PCR) testing: PCR testing is a molecular diagnostic method that can detect the DNA of A. ocellatum in fish samples. It is a highly sensitive and specific method that can provide accurate identification of the parasite.
Prevention of Red Velvet Disease
Preventing Marine Velvet Disease, caused by Amyloodinium ocellatum, requires effective management practices to reduce the risk of infection in fish populations. Here are some measures that can help prevent the disease:
Quarantine and acclimation: Quarantining newly acquired fish in a separate tank for observation and acclimation before introducing them into the main display tank can help prevent the introduction of parasites, including A. ocellatum, into the main tank. This allows for close monitoring of the fish for any signs of disease before they are introduced to the main tank.
Water quality management: Maintaining good water quality is crucial in preventing many fish diseases, including Marine Velvet Disease. Regular monitoring and maintenance of water parameters such as temperature, salinity, pH, ammonia, nitrite, and nitrate levels, along with appropriate filtration and water changes, can help create a healthy environment that is less conducive to parasites and other pathogens.
Fish health management: Ensuring that fish are in good health and stress-free can help prevent infections. Providing a balanced diet, avoiding overcrowding, and minimizing handling and other stressful events can help keep fish in optimal condition and less susceptible to diseases, including Marine Velvet Disease.
Equipment and tank hygiene: Regular cleaning and disinfection of aquarium equipment, including nets, heaters, and filters, can help prevent the buildup of pathogens, including A. ocellatum. Proper quarantine and isolation procedures for infected fish and thorough cleaning of tanks and equipment after an outbreak can help prevent the spread of the disease.
Avoiding introduction of contaminated water or livestock: Avoiding the introduction of water or livestock from known contaminated sources can help prevent the introduction of A. ocellatum or other parasites into the tank. Properly acclimating and quarantining any new additions to the tank can reduce the risk of disease transmission.
Treatment of infected fish: Promptly isolating and treating any fish showing signs of Marine Velvet Disease can help prevent the spread of the disease to other fish in the tank. Consultation with a qualified veterinarian or fish health professional for appropriate treatment options is recommended.
Marine Velvet Disease Treatment
The treatment of Amyloodinium ocellatum, commonly known as marine velvet disease or “velvet” in fish, typically involves a combination of environmental management and medication. Here are some common treatment options:
Quarantine and isolation: Infected fish should be promptly quarantined and isolated from the healthy fish to prevent the spread of A. ocellatum. This can help prevent further contamination of the aquarium or fish farm and reduce the risk of reinfection.
Water quality management: Maintaining optimal water quality is crucial in managing A. ocellatum infections. Regular monitoring and maintenance of water parameters such as temperature, salinity, pH, and ammonia levels can help create unfavorable conditions for the parasite and promote fish health.
Medications: There are several medications available for treating A. ocellatum infections, including antiparasitic drugs such as copper-based treatments (e.g., copper sulfate) and formalin-based treatments (e.g., formalin/malachite green). These medications can be effective in killing the trophonts and tomonts of A. ocellatum, but proper dosages, application methods, and treatment durations should be followed according to the manufacturer’s instructions and under the guidance of a veterinarian or experienced fish health professional.
Bath treatments: Some medications can be administered as baths, where infected fish are immersed in a diluted solution of the medication. Bath treatments can be effective in treating A. ocellatum infections, but care should be taken to follow proper dosages, duration, and acclimation procedures to avoid stress or harm to the fish.
Supportive care: Providing optimal nutrition, reducing stress, and maintaining a clean environment can also help improve the overall health and immunity of the infected fish, which may aid in their recovery from A. ocellatum infections.
Marine velvet vs ich
Marine velvet and ich, also known as ichthyophthiriasis, are both parasitic diseases that affect fish, but they are caused by different parasites and have some differences in their characteristics:
Causative Agents: Marine velvet is caused by a dinoflagellate parasite called Amyloodinium ocellatum, while ich is caused by a ciliated protozoan parasite called Ichthyophthirius multifiliis.
Appearance: Marine velvet is characterized by a yellow or gold dust-like appearance on the skin and gills of infected fish, which gives the fish a velvety or shimmering appearance. On the other hand, ich presents as small white spots, resembling grains of salt or sugar, on the skin, fins, and gills of infected fish.
Life Cycle: The life cycle of Amyloodinium ocellatum involves dinospores, trophonts, tomonts, and tomites, as described in a previous response. The life cycle of Ichthyophthirius multifiliis involves trophonts, tomonts, theronts, and cysts. Both parasites have complex life cycles involving both free-living and parasitic stages.
Host Range: Marine velvet can infect a wide range of marine and freshwater fish species, including both coldwater and tropical fish, whereas ich is primarily a freshwater disease that commonly affects freshwater fish, although it can also occur in marine fish in some cases.
Treatment: Treatment options for marine velvet and ich may vary, and different medications may be effective against each parasite. For marine velvet, treatments often involve antiparasitic medications such as copper-based or formalin-based treatments, while ich can be treated with medications such as malachite green, formalin, or copper-based treatments. Proper dosages, application methods, and treatment durations should be followed according to the manufacturer’s instructions and under the guidance of a veterinarian or experienced fish health professional for both diseases.
Environmental Management: Managing water quality, temperature, and other environmental factors is important for both marine velvet and ich. Maintaining optimal water quality, avoiding stressors such as poor water conditions or overcrowding, and providing proper nutrition can help improve fish health and immunity, and prevent the occurrence and spread of both diseases.
Researches on Amyloodinium ocellatum
Amyloodinium ocellatum, commonly known as AO, is an ectoparasite protozoan that causes amyloodiniosis in European seabass (Dicentrarchus labrax).
Despite being a major threat to marine aquaculture in the Mediterranean region, there is limited knowledge about the basic molecular biology of AO and its interactions with the host.
In a studies, researchers have used advanced techniques such as de novo transcriptome sequencing and RNA-seq to uncover unique aspects of AO’s biology and its interaction with the host.
A study conducted de novo transcriptome sequencing of AO tomonts, the early stage of the parasite’s life cycle. Tomonts were purified from infested seabass gills and their total RNA was processed and sequenced using Illumina sequencer.
The resulting transcriptome assembly revealed that a significant portion (56.12%) of the contigs belonged to dinophyceae, a class of dinoflagellates to which AO belongs.
Further functional annotations of the contigs indicated the presence of several peptidases, which are enzymes that play important roles in various biological processes.
Additionally, a BLAST search for known virulent factors from the virulence database identified hits to various proteins such as Rab proteins, Ribosomal phosphoprotein, Heat-shock protein 70, Casein kinases, Plasmepsin IV, and Brucipain, which may be involved in AO’s virulence.
Another study investigated the immune response of European seabass during AO infestation using RNA-seq and real-time PCR. The researchers compared gene expression between gills and head kidney of AO-infested seabass and uninfested seabass.
They found that genes related to the immune system, such as perforin and CC1, were upregulated in the gills, while genes involved in interferon (IFN) related pathways were upregulated in the head kidney.
Subsequent validation of the differentially expressed genes using real-time PCR revealed that cytokines (CC1, IL-8) and antimicrobial peptide (Hep) were strongly stimulated during the early infestation stage and gradually reduced during the recovery stage.
Immunoglobulin (IgM) expression was also higher during the recovery stage, indicating a potential role of adaptive immunity in combating AO infestation.
In-situ hybridization showed positive signals of CC1 mRNA in AO-infested gills, further supporting the involvement of chemokines in the immune response to AO infestation in seabass.
Interestingly, another study used histological and molecular techniques to confirm the presence of AO in infected seabass gills and investigate the immune response of the host.
Histological observation of infected gill samples revealed the presence of AO trophonts anchored to the oro-pharyngeal cavity. Molecular analysis using small subunit (SSU) rDNA confirmed the presence of AO in the gills.
Immunohistochemical labeling of immune-related genes in infected gills showed positive signals for igm, inos, pcna, and cytokeratin, indicating the activation of innate immunity and cell proliferation in response to AO infestation.
Overall, these studies shed light on the unique biology of Amyloodinium ocellatum and its interaction with the host. The use of advanced molecular techniques has provided valuable insights into AO’s transcriptome, virulence factors, and the host immune response during infestation.
Further research in this area may contribute to the development of novel diagnostics and vaccines for managing AO infestation in European seabass and other susceptible fish species.
References
- Francis-Floyd, Ruth, and Maxine R. Floyd. Amyloodinium ocellatum, an important parasite of cultured marine fish. Stoneville, MS, USA: Southern Regional Aquaculture Center, 2011.
- Picón-Camacho, S. M., Thompson, W. P., Blaylock, R. B., & Lotz, J. M. (2013). Development of a rapid assay to detect the dinoflagellate Amyloodinium ocellatum using loop-mediated isothermal amplification (LAMP). Veterinary parasitology, 196(3-4), 265–271. https://doi.org/10.1016/j.vetpar.2013.04.010
- Bessat, M., & Fadel, A. (2018). Amyloodiniosis in cultured Dicentrarchus labrax: parasitological and molecular diagnosis, and an improved treatment protocol. Diseases of aquatic organisms, 129(1), 41–51. https://doi.org/10.3354/dao03237
- Massimo, M., Volpatti, D., Galeotti, M., Bron, J. E., & Beraldo, P. (2022). News Insights into the Host-Parasite Interactions of Amyloodiniosis in European Sea Bass: A Multi-Modal Approach. Pathogens (Basel, Switzerland), 11(1), 62. https://doi.org/10.3390/pathogens11010062
- Dhayanithi, N. B., Sudhagar, A., Kumar, T. T. A., & Lal, K. K. (2022). Study on amyloodiniosis outbreak in captive-bred percula clownfish (Amphiprion percula) and improved control regimens. Journal of parasitic diseases : official organ of the Indian Society for Parasitology, 46(4), 1103–1109. https://doi.org/10.1007/s12639-022-01530-1
- Moreira, M., Soliño, L., Marques, C. L., Laizé, V., Pousão-Ferreira, P., Costa, P. R., & Soares, F. (2022). Cytotoxic and Hemolytic Activities of Extracts of the Fish Parasite Dinoflagellate Amyloodinium ocellatum. Toxins, 14(7), 467. https://doi.org/10.3390/toxins14070467
- Moreira, M., Herrera, M., Pousão-Ferreira, P., Navas Triano, J. I., & Soares, F. (2018). Stress effects of amyloodiniosis in gilthead sea bream Sparus aurata. Diseases of aquatic organisms, 127(3), 201–211. https://doi.org/10.3354/dao03199
- Kuperman, B. I., & Matey, V. E. (1999). Massive infestation by Amyloodinium ocellatum (Dinoflagellida) of fish in a highly saline lake, Salton Sea, California, USA. Diseases of aquatic organisms, 39(1), 65–73. https://doi.org/10.3354/dao039065
- Moreira, M., Schrama, D., Soares, F., Wulff, T., Pousão-Ferreira, P., & Rodrigues, P. (2017). Physiological responses of reared sea bream (Sparus aurata Linnaeus, 1758) to an Amyloodinium ocellatum outbreak. Journal of fish diseases, 40(11), 1545–1560. https://doi.org/10.1111/jfd.12623
- Byadgi, O., Beraldo, P., Volpatti, D., Massimo, M., Bulfon, C., & Galeotti, M. (2019). Expression of infection-related immune response in European sea bass (Dicentrarchus labrax) during a natural outbreak from a unique dinoflagellate Amyloodinium ocellatum. Fish & shellfish immunology, 84, 62–72. https://doi.org/10.1016/j.fsi.2018.09.069
- Byadgi, O., Massimo, M., Dirks, R. P., Pallavicini, A., Bron, J. E., Ireland, J. H., Volpatti, D., Galeotti, M., & Beraldo, P. (2021). Innate immune-gene expression during experimental amyloodiniosis in European seabass (Dicentrarchus labrax). Veterinary immunology and immunopathology, 234, 110217. https://doi.org/10.1016/j.vetimm.2021.110217
- Byadgi, O., Marroni, F., Dirks, R., Massimo, M., Volpatti, D., Galeotti, M., & Beraldo, P. (2020). Transcriptome Analysis of Amyloodinium ocellatum Tomonts Revealed Basic Information on the Major Potential Virulence Factors. Genes, 11(11), 1252. https://doi.org/10.3390/genes11111252
