Ichthyobodosis, also known as costiasis, is a common parasitic disease caused by the protozoan parasite Ichthyobodo necator affects gills of fish, leading to various clinical signs and health issues in aquaculture and ornamental fishkeeping.
This comprehensive review aims to provide an in-depth understanding of the causes, symptoms, and treatment options for ichthyobodosis.
The article will cover the life cycle and transmission of the parasite, clinical manifestations and symptoms of ichthyobodosis in fish, and various treatment approaches including chemical and environmental management strategies.
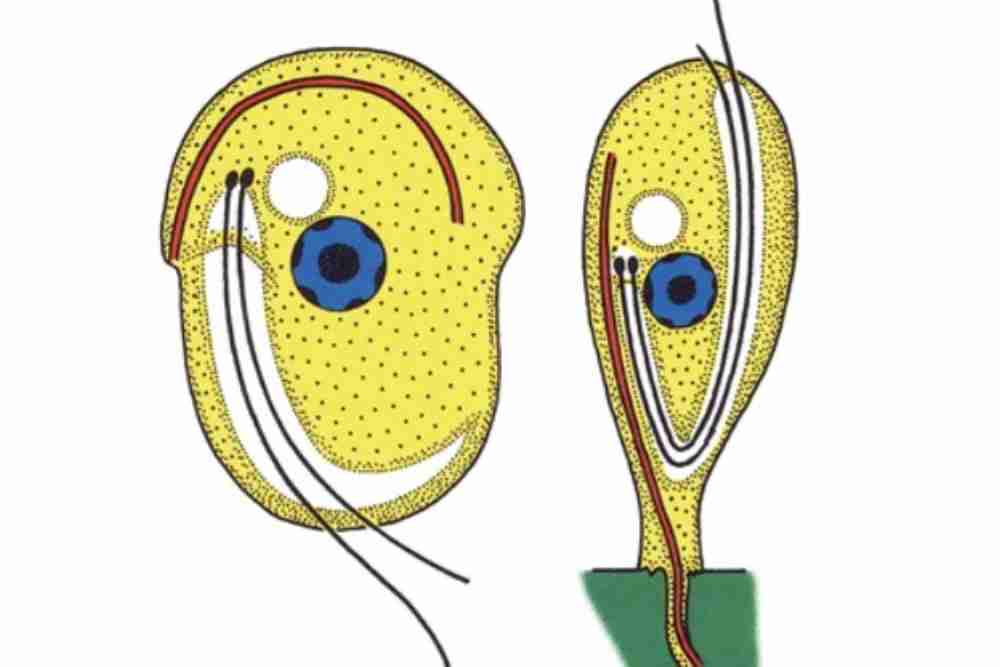
Causes of Ichthyobodosis (Costiasis)
Ichthyobodosis is a fish disease caused by Ichthyobodo necator (Costia), the parasite responsible for ichthyobodosis in freshwater fishes. The early name of the disease, Costia necatrix.
Ichthyobodo necator is regarded primarily as a freshwater fish ectoparasite with a broad host range. Ichthyobodo necator is a protozoan parasite.
Ichthyobodo necator was first described by L. F. Henneguy in 1883 and more fully in 1884 and he named it Bodo necator.
Body Characteristics of Ichthyobodo necator
These parasites are microscopic sizes vary from 5-6 micrometers. Ichthyobodo necator often found infesting fish’s skin and gills, including salmonids.
These parasites can reduce the ability of young salmon to adapt to seawater if it invests in the gill if it infests in the gill.
Free swimming form is oval to spherical and measures 5-8 micrometers.
Its pre division stage has three and four flagella, and larger stages have two.
Attaches with a host by its pointed anterior end.
In adverse environmental conditions, they form a cyst, which becomes an additional infection source. They process a ventral flat disc.
It is an obligate parasite, meaning that it can only survive by living inside another organism.
Geographical distribution of Ichthyobodo necator
Marine Ichthyobodo necator has a worldwide distribution, like Ichthyobodo necator in freshwater. The parasite has been found in the Pacific, Atlantic, and adjacent seas, including Australian water.
These ectoparasitic protozoans were widespread at water temperatures between 2 degrees to 15 degrees Celsius.
The earliest documented case of Ichthyobodo sp. infections in fish from a marine environment was identified in juvenile Chinook salmon.
Heavy parasite infestation frequently occurs in hatchery-reared salmon in Northern Japan.
Fish species affected by Ichthyobodo necator
Infection by Ichthyobodo necator have recorded more than 60 different host species in both freshwater and marine water.
- Atlantic cod
- Salmon
- Trout
- Cyprinus carpio
- Haddock
- Turbot
- Halibut
- Spotted wolffish
- Clarias gariepinus
- Flounder
- Mullet
- Tilapia
Susceptible Host\Species Infected
This parasite does not distinguish a parasitic host. This creature has spread into the oceans where it breeds with freshwater varieties.
Juvenile fish (fry and fingerlings) and adults are believed to be particularly susceptible to the attack.
Ichthyobodo necator (Costia) Life cycle
- Parasite attach to the body by attachment organ and the body is pyriform.Its free non-feeding form is oval shaped which has two or rarely four flagella.
- The parasitic stage found in fish bodies is known as trophont.
- Parasitic trophont develops a suitable size, drops off from the host body, becomes a cyst, and turns into a tomont stage.
- Within the cyst, tomont divides into 10 to 11 divisions to produce small tomites.
- Tomites break the cyst wall to become theronts.
- Theronts are the infective stages that affect the host.
Ichthyobodo necator (Costia) Symptoms
Fish infected with Ichthyobodo are frequently languid and listless and typically exhibit flashing behavior.
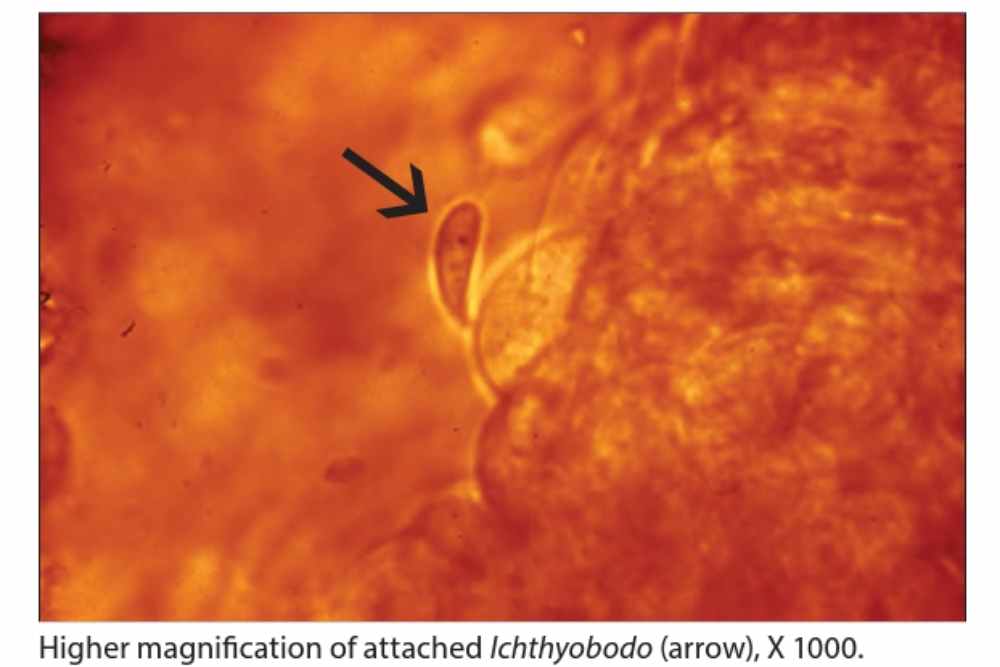
More advanced cases are characterized by a blue-gray film on the surface of fish owing to elevated mucus generation and and hyperplasia of the epidermal epithelium.
Gill hyperplasia and lamellar fusion (clubbing) are signs of ichthyobodosis, a disease caused by infestation of the gills by parasites. Secondary bacterial and fungal infections are common in fish with ichthyobodosis.
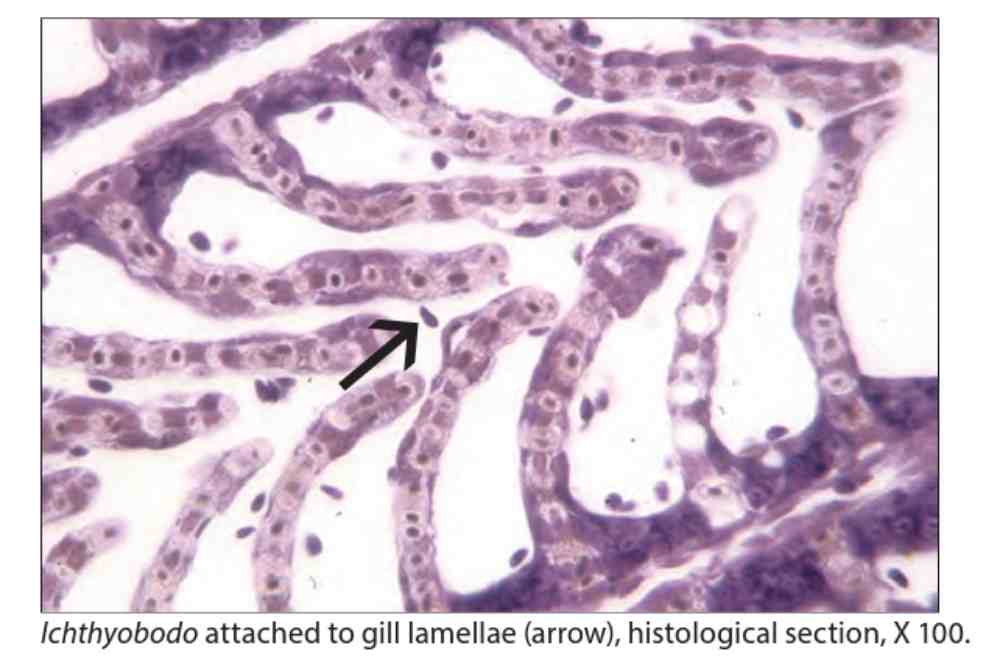
In salmon researcher found that the destruction of the surface of the infected cells show noticeable vacuolation of the cells, but the nucleus usually remains unharmed. It is concluded that I. necator does not eat the cell nucleus.
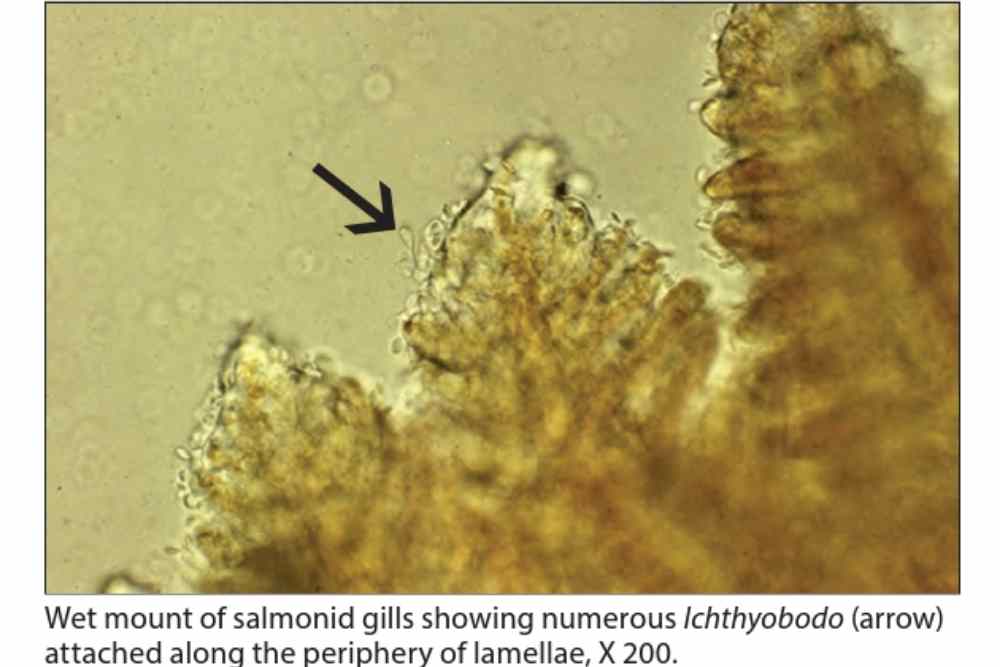
This parasitic caused hyperplasia of the malphigian and goblet cells, followed by form and function of the epidermis below infection, followed by the formation of plaques above the basement membrane.
Then the plaques detaching, leaving the epidermis underneath a layer of cells sticking to the basement membrane.
Cell kinetic studies showed that I. necator caused the cells immediately below infestations to divide, a markedly different pattern from that of normal teleost epidermal cell proliferation.
The possibility that the parasite secretes some form of digestive enzyme is postulated.
In areas where sloughing had occurred, the remaining malphigian cells were seen to be in the process of division (Robertson et. al., 1981).
Ichthyobodo necator Transmission
The disease affects both farmed and wild fish. These protozoan parasites are typically transmitted from fish to fish by direct contact, or through contaminated water.
Sub clinically parasitized fish are the reservoirs for the parasite in the environment, and can infect other fish without showing any symptoms themselves.
The transmission of ichthyobodosis is a serious problem for the aquaculture industry, as it can lead to mass die-offs of fish in farms.
The host is infected within an hour after division or the parasite dies.
Ichthyobodo necator (Costia) Diagnosis
The diagnosis of Ichthyobodo necator, a parasitic protozoan that infects fish, can be definitively made through various methods. One common method is through wet mount skin and gill preparations, where the parasite can be observed under a microscope.
Ichthyobodo necator typically exhibits an asymmetrical, oval body in its attached form, with fewer free-swimming forms that are more ellipsoidal in shape.
Sometimes, two unequal flagella can be seen from the anterior end, which lie along a funnel-shaped groove on the ventral side of the parasite. In stained histological sections, the attached forms of the parasite can also be detected.
Two of the creature’s flagella have unequal lengths and lie in a groove along a funnel-shaped near the bottom and if look closely, they can be noticeable as blobs in stained histological sections.
There are certain characteristics that can aid in the diagnosis of Ichthyobodo necator. For instance, when the parasite turns its crescent-shaped body, the free form of the parasite may exhibit flickering motion.
However, in heavy infestations, detecting attached parasites can be more challenging. In such cases, adjusting the magnification to the edge of the gill epithelium where the parasites form a palisade may help in locating them.
In addition to microscopic examination, Polymerase Chain Reaction (PCR) can also be used for the diagnosis of Ichthyobodo necator.
PCR is a highly sensitive and effective method that can detect specific DNA sequences unique to Ichthyobodo necator, providing a reliable means of identification.
Ichthyobodo necator (Costia) Preventive
Preventing Ichthyobodosis, a disease caused by protozoan parasites, can be achieved through several key preventive measures to ensure the fish are kept in a healthy and stress-free environment with robust immune systems.
First and foremost, providing a stress-free environment for the fish is crucial in preventing Ichthyobodosis.
This can be achieved by minimizing environmental stressors such as sudden changes in water temperature, poor water quality, overcrowding, and inadequate nutrition.
Maintaining stable water conditions and providing appropriate tank or pond size for the fish species are essential to keep the fish healthy and less susceptible to diseases.
Regular cleaning of the aquarium, pond, or tank is also important in preventing the buildup of organic debris that can serve as a breeding ground for parasites. Excess food, uneaten food, and fish waste should be removed promptly to prevent water contamination and the spread of parasites.
Proper feeding practices are also critical in preventing Ichthyobodosis. Feeding the fish the correct amount of food and avoiding overfeeding can prevent excess organic debris in the water, reducing the chances of parasite infestation.
Avoiding overstocking of fish in a tank or pond is another preventive measure. Overcrowding can lead to increased stress, poor water quality, and higher chances of disease outbreaks.
Ensuring that the fish population does not exceed the capacity of the holding tank or pond can help prevent the spread of diseases like Ichthyobodosis.
Quarantining new fish before introducing them to an established tank or pond is also recommended.
Quarantine periods allow for observation and potential treatment of any potential diseases or parasites that may have been introduced with new fish, preventing the spread of diseases to the existing fish population.
Regular maintenance, including water changes, filter cleaning, and equipment inspection, should be conducted to ensure optimal water quality and a healthy environment for the fish.
Regularly testing the water for abnormalities such as pH, temperature, ammonia, nitrite, and nitrate levels can help detect any potential issues early and take appropriate corrective actions.
Ichthyobodo necator (Costia) Treatment
- Formalin (flash treatment) 166 ppm for 1 hour, bath 1:4000 for 15-20 mins is well tolerated. Treatment for 1 hour with formalin 1:6000 is also very effective.
- Ponds with infected fish are drained, and quicklime, chloride, or limes are added before restocking.
- Exposing fingerlings to a weak solution of malachite green (1:400000) for 40-60 mins
- Cyprinids lose their infection when dipped daily in copper sulfate (500 ppm) for 1-2 mins, or salt solutions (5%) are recommended for larger fish.
- Other chemicals like methyl blue, potassium permanganate, Lysol, etc. can be used.
References
- Savage, J. (1935). Notes on costiasis. Transactions of the American Fisheries Society, 65(1), 332-333.
- Fish, F. F. (1941). Notes on Costia necatrix. Transactions of the American Fisheries Society, 70(1), 441-445.
- Bhatti, M. N. (1979). A note on the occurrence of costiasis disease in the stinging catfish, Heteropneustes fossilis (Bloch) from Basrah waters. Arab Gulf, 11(1), 216.
- Ellis, A. E., & Wootten, R. (1978). Costiasis of Atlantic salmon. Salmo salar, 389-393.
- Poppe, T. T., & Hastein, T. (1982). Costiasis in salmon smolt (Salmo salar L.) in sea water. Norsk Veterinaertidsskrift, 94, 259-262.
- Haastein, T., & Poppe, T. (1984). Costiasis, an old disease in a new environment [Ichtyobodo necator]. Aktuelt fra Statens Fagtjeneste for Landbruket (Norway).
Iashchuk, V. D. (1977). Control of costiasis of the trout. Veterinariia, (9), 71-73. - Kanaev, A. I., & Vlasenko, M. I. (1973). Measures for the control of costiasis in carp during the winter. Veterinariia, 11, 69-70.
- Naich, M., & Bilqees, F. M. (1998). The Incidence of Costiasis on Different Rainbow Trout Fish Farms on the River Itchen and Test Hampshire, UK. PROCEEDINGS OF PARASITOLOGY, 19-25.
- Roubal, F. R., Bullock, A. M., Robertson, D. A., & Roberts, R. J. (1987). Ultrastructural aspects of infestation by Ichthyobodo necator (Henneguy, 1883) on the skin and gills of the salmonids Salmo salar L. and Salmo gairdneri Richardson. Journal of Fish Diseases, 10(3), 181-192.
- Franke, J. (1908). Radical prevention of Costia necatrix in salmonoid fry.
- Alvarez-Pellitero, P. (2004). Report about fish parasitic diseases. Etudes et Recherches, Options Mediterranennes. CIHEAM/FAO, Zaragoza, 103-130.
- Egidius, E. (1983). An overview of health problems in marine fish culture in temperate waters. Rapports et Proces-Verbaux des Reunion, Conseil International pour l’Exploration de la Mer, 182, 33-36.
- Tavolga, W. N., & Nigrelli, R. F. (1947). Studies on Costia necatrix (Henneguy). Transactions of the American Microscopical Society, 66(4), 366-378.
- Robertson, D. A., Roberts, R. J., & Bullock, A. M. (1981). Pathogenesis and autoradiographic studies of the epidermis of salmonids infested with Ichtyobodo necator (Henneguy, 1883). Journal of Fish Diseases, 4(2), 113-125.
- Ogut, H., & Akyol, A. (2007). Prevalence and intensity of ectoparasites in rainbow trout (Oncorhynchus mykiss) from larvae stage to market size in Turkey.
- Grizzle, J. M. (2000). Introduction to fish gill histopathology.
- Speare, D. J. (2002). Non-infectious disorders of coldwater fish. Diseases and disorders of finfish in cage culture, 171-193.
- Urawa, S., Ueki, N., & Karlsbakk, E. (1998). A review of Ichthyobodo infection in marine fishes. Fish Pathology, 33(4), 311-320.
- Blackstock, N. (1985). Factors affecting the structure of salmonid epidermis.
- Robertson, D. A. (2019). A review of Ichthyobodo necator (Henneguy, 1883) an important and damaging fish parasite. Recent advances in aquaculture, 1-30.
- Lamas, J., & Bruno, D. W. (1992). Observations on the ultrastructure of the attachment plate of Ichthyobodo sp., from Atlantic salmon, Salmo salar L., reared in the marine environment. BULLETIN-EUROPEAN ASSOCIATION OF FISH PATHOLOGISTS, 12, 171-171.
- Robertson, D. A. (1979). Host‐parasite interactions between Ichtyobodo necator (Henneguy, 1883) and farmed salmonids. Journal of Fish Diseases, 2(6), 481-491.
- Valheim, M., Håstein, T., Myhr, E., Speilberg, L., & Ferguson, H. W. (2000). Varracalbmi: a new bacterial panophthalmitis in farmed Atlantic salmon, Salmo salar L. Journal of Fish Diseases, 23(1), 61-70.
- Todal, J. A., Karlsbakk, E., Isaksen, T. E., Plarre, H., Urawa, S., Mouton, A., & Nylund, A. (2004). Ichthyobodo necator (Kinetoplastida) a complex of sibling species. Diseases of Aquatic Organisms, 58(1), 9-16.
- Fisher, F. S. (1940). Formalin for external protozoan parasites: A report on the prevention and control of Costia necatrix. Progressive Fish-Culturist, 7(48), 1-10.
- LAMA, J., & BRUNoº, D. W. (1992). MENT PLATE OF ICHTHYOBODO SP., FROM ATLANTIC SALMON, SALMO SALAR L., REARED IN THE MARINE ENVIRONMENT. Bull. Eur. Ass. Fish Pathol, 12(5), 171.
- Mitchell, S. O., & Rodger, H. D. (2011). A review of infectious gill disease in marine salmonid fish. Journal of fish diseases, 34(6), 411-432.