As an avid fish enthusiast, it’s important to be aware of the common parasitic infestation called gill flukes, caused by Dactylogyrus, that can affect fish health and well-being.
In this discussion, we will delve into the causes, symptoms, and treatment options for gill flukes, providing you with valuable knowledge to help you identify, prevent, and effectively treat this condition in your beloved aquatic pets.
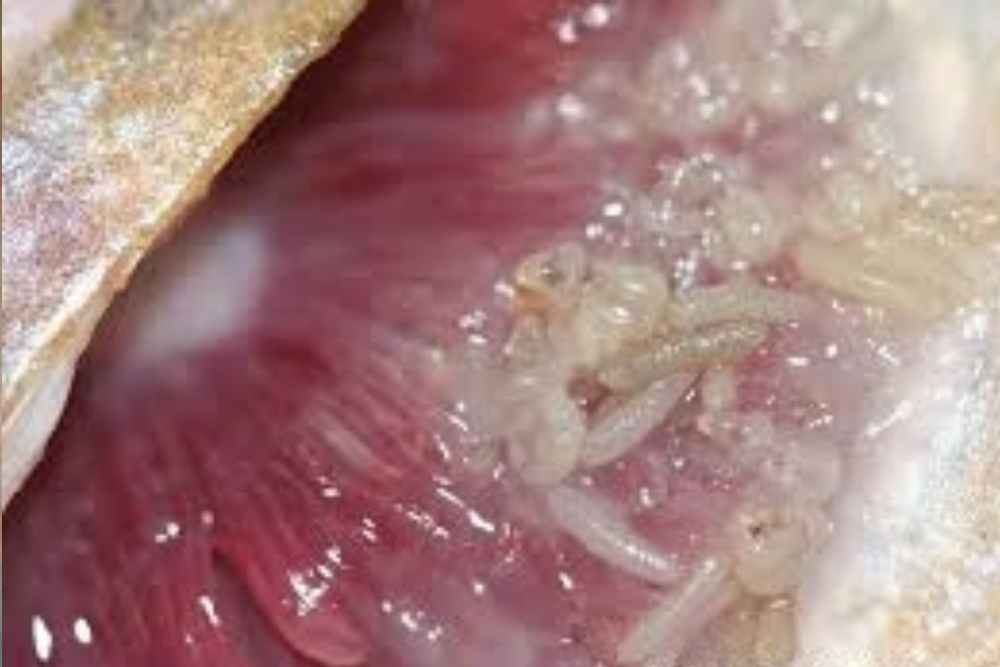
Causes of Gill Flukes
Gill fluke is a monogeneans parasite, commonly infects the gills of marine and freshwater fish. Dactylogyrus sp is a group of monogenean gill parasites that are highly specific to freshwater fish of the family Cyprinidae.
Dactylogyrus is a highly diverse genus with more than 900 described species that primarily parasitize the gills of Cyprinidae. They are ectoparasites with a direct life cycle.
Classification of Dactylogyrus
- Kingdom: Animalia
- Phylum: Platyhelminthes
- Class: Monogenea
- Order: Monopisthocotylea
- Family: Dactylogyridae
- Genus: Dactylogyrus
- Scientific name: Dactylogyrus spp.
Different Dactylogyrus Species
Some of the Dactylogyrus species found worldwide are:
- Dactylogyrus vastator
- Dactylogyrus extensus
- Dactylogyrus gracilis
- Dactylogyrus anchoratus
- Dactylogyrus intermedius
- Dactylogyrus lamellatus
- Dactylogyrus minutus
- Dactylogyrus sphyrna
- Dactylogyrus baueri
- Dactylogyrus solidus
Characteristics of Dactylogyrus
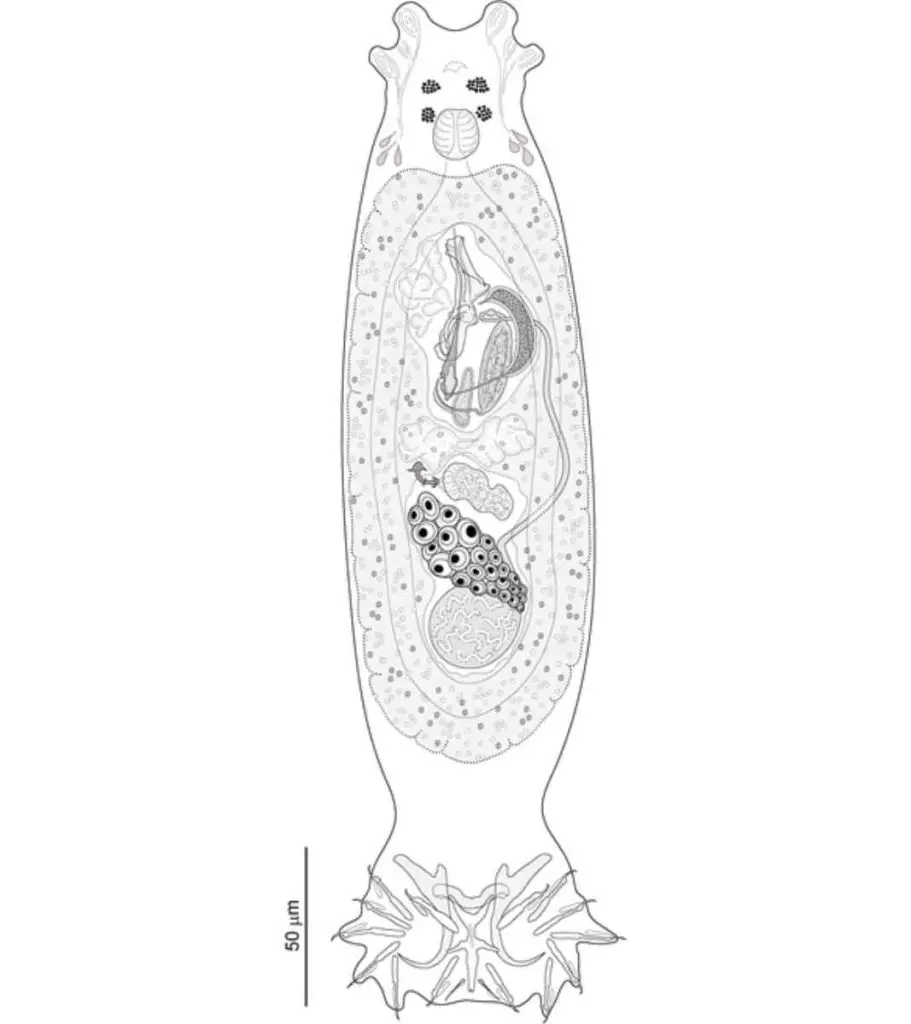
Gill flukes, scientifically known as Dactylogyrus, are parasitic flatworms that infest the gills of fish.
These flukes have distinct characteristics that include a dorso-ventrally flattened body with bilateral symmetry, resembling a leaf shape, and measuring around 7 to 8 mm in length.
They have two pairs of anchors, four eye spots, and seven pairs of marginal hooks.
The structure of gill flukes also includes one to two connective bars and two spindle-shaped needles in their seminal vesicle.
They possess an oral sucker around their mouth and a ventral sucker for attachment, known as a haptor.
Gill Flukes are hermaphrodite, meaning they have both male and female reproductive organs, and they are oviparous, laying eggs for reproduction.
The alimentary canal of gill flukes is well developed, with a muscular pharynx and esophagus for feeding. They also have flame cells, which serve as excretory organs.
These characteristics make Gill Flukes a complex and specialized parasite that can cause harm to the gills of fish, leading to various health issues. Understanding the anatomy and biology of Gill Flukes is crucial in identifying and treating infestations in fish populations.
Life Cycle of Dactylogyrus spp.
Its life cycle involves only one host, the fish, and is relatively simple compared to other parasitic infections. Here’s a more detailed explanation of the life cycle of Dactylogyrus sp. broken down into stages:
1. Egg stage
Adult Dactylogyrus parasites attach themselves to the gills of a freshwater fish and lay eggs. These eggs are attached to the gill filaments or the surrounding tissue.
2. Oncomiracidium stage
The eggs hatch into free-swimming larvae called oncomiracidia. Oncomiracidia has a ciliated epidermis and is equipped with hooks and suckers that allow them to attach to the fish host’s gills.
3. Adulthood stage
Oncomiracidia attaches themselves to the host fish’s gills and develops into adult parasites. The adult Dactylogyrus parasites feed on the host’s blood and tissues, using their hooks to anchor themselves to the gill filaments.
4. Reproduction stage
The adult parasites continue to lay eggs, which either remain attached to the gill filaments or are released into the water.
5. Completion stage
The life cycle of Dactylogyrus sp. is completed when the eggs hatch into oncomiracidia, which then attach to new hosts and begin the cycle again.
Overall, the life cycle of Dactylogyrus sp. involves only one host, the freshwater fish, and can be completed relatively quickly, leading to heavy infestations in fish populations if not controlled.
Geographical Distribution of Dactylogyrus
These species are widely distributed across the world and can be found in freshwater fish on every continent except Antarctica.
The specific distribution is determined by the fish host they infect, as well as the environmental conditions of the water in which the fish live.
For example, D. vastator and D. intermedia are commonly found on the gills of carp and other Cyprinid fish in Europe and Asia, while D. lamellatus is found in both North America and Europe that infecting the gills of various fish species such as trout, bass, and catfish.
D. anchoratus is commonly found in Russia and infects the gills of char and grayling, while D. solidus is found in Africa and primarily infects the gills of tilapia.
The overall geographical distribution of Dactylogyrus sp coincides with the area of the natural distribution of Cyprinids, which includes Africa, Asia, North America, Europe, and Eurasia.
Fish Species Infected by Dactylogyrus
Affected fishes are- Cyprinus carpio, Symphysodon discus, Pterophyllum scalare, Pangasius macronema, Carassius auratus (goldfish), Chaetodon xanthurus, Scatophagus argus, scirrhous mrigala, ctenopharyngodon Idella,Oreochromis niloticus, etc.
Factors Accelerate the Presence of Dactylogyrus spp
Several factors can accelerate the presence and growth of Dactylogyrus spp. in fish populations. These include:
1. Overcrowded conditions
Overcrowding of fish in ponds, tanks, and fish farms can make them more susceptible to Dactylogyrus spp. infestations. Crowded conditions lead to increased stress on fish, reducing their immunity to diseases and parasites.
2. Warm water temperatures
Dactylogyrus spp. infestations tend to increase during warmer water temperatures. High water temperatures accelerate the life cycle of these parasites, increasing their population in fish gills.
3. Poor water quality
Poor water quality can promote the growth and breeding of Dactylogyrus spp. and other parasites. High levels of nutrients, organic matter, and pollutants in water bodies can create favorable conditions for parasites to thrive.
4. Introduction of infected fish
The introduction of infected fish into a water body can accelerate the presence of Dactylogyrus spp. in fish populations. Infected fish can spread the parasites to other fish in the same water body.
5. Confinement of fish
When fish are confined within small spaces, such as ponds or tanks, they are more likely to be exposed to a higher concentration of parasites, including Dactylogyrus spp.
Gill Fluke Symptoms
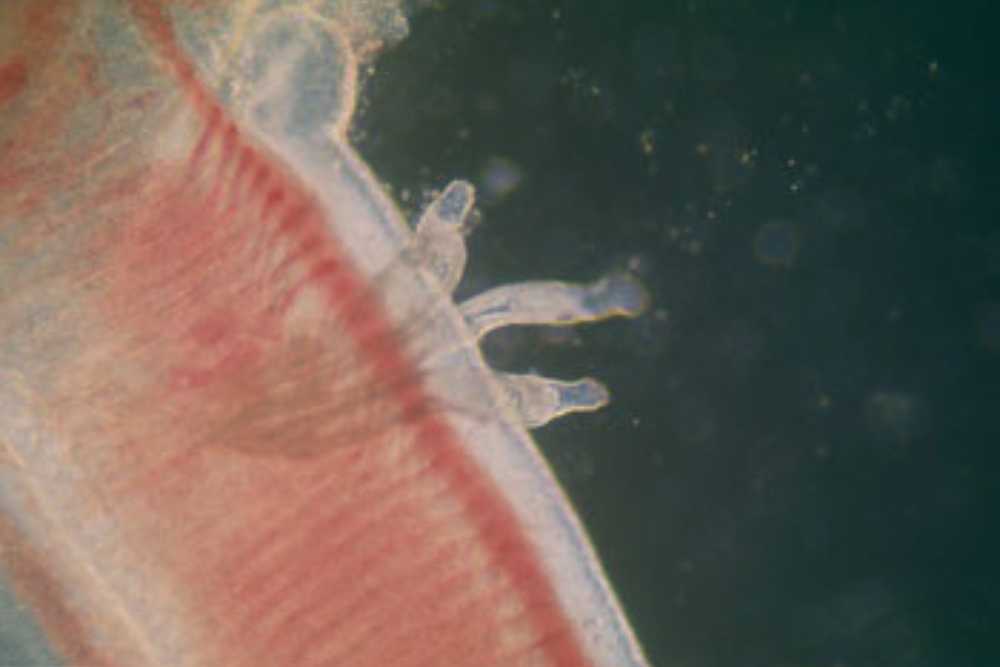
The fish may become restless and swim towards the water’s surface in an attempt to obtain oxygen, as they may struggle to respire properly due to gill damage.
The gills themselves may become swollen and pale in color, and there may be an increase in mucous secretion, leading to a high mucous coating on the gills.
–The fish may also lose weight and appear thin, and the edges of the gills may take on a grayish hue.
-In severe cases, the fish may exhibit open operculum (gill covers) as they struggle to breathe.
-Dark coloration, often appearing as patches or spots, may also be noticeable among the diseased fish.
–Affected fish may lose their appetite and cease to feed, and may exhibit abnormal behavior such as swimming near the water’s surface and jumping out of the water in an attempt to obtain more oxygen.
-These symptoms collectively indicate a potentially serious health issue for the fish, likely caused by gill fluke infestation.
Gill Flukes Diagnosis
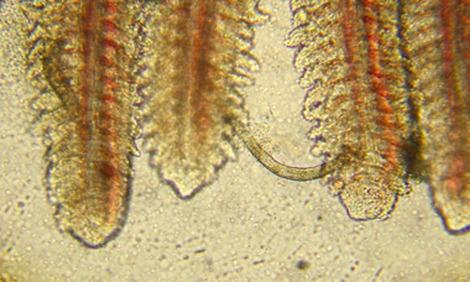
Gill Flukes attaches to the host’s gill epithelium with hooks on their posterior, where they feed on mucus and blood. Infestations may lead to secondary infections with other pathogens.
The diagnosis of Dactylogyrus spp. infestations in fish can be done through different methods, including:
A physical observation: Physical observation is needed to observe any diseases.
Gill smear
Remove part of a gill arch with scissors and holding the tissue in forceps, smear the lamellae on a microscope slide. The material adhering to the slide may contain mucus, epithelial cells, and blood cells of the gills.
Allow the smear to air dry and pass the slide through an open flame 2-3 times fixing the smear on the slide.
If parasites are found, it will compare with the characteristics of Dactylogyrus sp. Then, it will be sure what sort of parasite caused the disease.
Macroscopic examination
The presence of Dactylogyrus spp. can be seen through the naked eye. The adult parasites appear as tiny, white, or yellowish specks, often clinging to the gills of the fish.
The presence of brownish-black spots on the gills or bleeding and reddening on the gills and body of the fish also indicates an infestation.
PCR tests
Polymerase Chain Reaction (PCR) is a molecular diagnostic method that detects the DNA of the Dactylogyrus spp. from infected fish samples. PCR is highly sensitive, reliable and sensitive diagnostic methods are needed for detecting infections in various fish species.
To overcome this problem, the multiplex polymerase chain reaction (m-PCR) assay to rapidly identify and diagnose Dactylogyrus spp. simultaneously on the target genes in field-collected samples under one reaction.
ELISA tests
Enzyme-Linked Immunosorbent Assay (ELISA) is another diagnostic test that can detect the Dactylogyrus spp. antigen in the fish samples.
Gill Fluke Prevention
To prevent gill fluke infestations in fish, several measures can be taken:
Maintaining good water quality and pH: Regular monitoring and maintenance of water quality parameters such as temperature, pH, ammonia, nitrite, and nitrate levels can help create an environment that is less conducive to the proliferation of fish parasites, including gill flukes.
Increasing water exchange: Frequent water exchanges, either through partial water changes or continuous water flow, can help dilute and remove potential sources of gill fluke infestation, reducing the risk of infection.
Increasing aeration: Proper aeration, such as the use of aerators, air stones, or surface skimmers, can help increase oxygen levels in the water and create a more oxygen-rich environment for the fish. This can help the fish maintain healthy gill function and reduce stress, making them less susceptible to gill fluke infestations.
Reducing organic load: Excess organic matter in the water, such as uneaten food, decaying plants, and fish waste, can contribute to poor water quality and provide a breeding ground for parasites. Regular removal of debris, proper waste management, and avoiding overfeeding can help reduce the organic load in the water and minimize the risk of gill fluke infestations.
Liming: The application of lime (calcium carbonate) to the water can help raise the pH and create a less favorable environment for gill flukes to thrive. However, the appropriate dosage and application method should be carefully followed to avoid any adverse effects on fish or other aquatic organisms.
Responsible stocking density: Overcrowding of fish can lead to stress, poor water quality, and increased susceptibility to diseases, including gill fluke infestations. Ensuring that fish are stocked at an appropriate density based on the size of the tank or pond and the species being kept can help prevent gill fluke outbreaks.
Gill Flukes Treatment
Dactylogyrus is a type of parasitic flatworm that commonly affects fish in aquariums and ponds. It can cause several health problems, including damage to the gills, loss of appetite, and stunted growth. The following are some treatment options for Dactylogyrus spp.
Salt baths: One effective treatment for Dactylogyrus is a salt bath. Dissolve aquarium or pond salt in water and place your fish in the solution for several minutes. The concentration of salt should be based on the type of fish you have, so it’s best to consult with a veterinarian or experienced fish keeper for the appropriate dosage.
Formalin treatment: Formalin is a remedy in many cases used to treat parasitic infections in fish. Follow the dosage guidelines carefully and examine the fish intently for any damaging reactions.
Potassium permanganate treatment: It has been used as spray applied to a part of the pond about 10 feet away from the banks and calculated to produce a concentration of 10 ppm. Baths of indeterminate duration were effective at 2 ppm and 3-5 ppm, single application. Monogenea can be eliminated by treatment with various disinfectants.
Improve water quality: Poor water can make contributions to the improvement and unfolding of Dactylogyrus infections. Maintain correct water first-rate 9 with the useful resource of performing ordinary water changes, holding the water pH within the amazing range, and making certain enough filtration.
Cleaning: Regular cleaning of the aquarium or pond can help prevent Dactylogyrus infestations. Remove any uneaten food and feces from the water, and use a water filter to keep the water clean.
Quarantine: Infected fish should be quarantined to prevent the spread of Dactylogyrus to other fish in the aquarium or pond. Treat the infected fish in a separate tank before returning them to the main tank.
Malachite green: Malachite green can be sprayed in the pond to give a concentration of 0.15 ppm, and the treatment is to be continued for three days.
Anthelmintic drugs: To governance this parasite effectively, the anthelmintic properties of several extracts obtained from Euphorbia fischeriana and four common anthelmintic drugs (praziquantel, trichlorfon, mebendazole, 40% phoxim) against adults and eggs of Dactylogyrus vastator can be used. Rosemary (Rosmarinus officinalis) is a common medicinal herb, having antimicrobial and antitumor properties.
| Compound | Treatment method and dose rate |
| Formalin (40% formaldehyde) | Bath: 20-30 minutes, 100-250 ppmBath: permanent, 25-50 ppm |
| Potassium permanganate (KMnO₄) | Bath: permanent, 2 ppmRepeated treatment may be necessary |
| Salt | Bath: Indefinite, 0.1-0.2% Bath: 20-30 min, 3% |
| Malachite Green/ Formalin mixture | Bath: Indefinite, 0.1ppm malachite green & 25 ppm |
References
- Das, D. R., & Chandra, K. J. (2018). Fish parasitology, mymensingh, Bangladesh.
- Das, D. R., & Chandra, K. J. (2018). Seasonal variation of gill, skin, muscle, liver and kidney pathology of mrigal (Cirrhinus cirrhosus) in cultural pond fisheries, mymensingh, Bangladesh. Bangladesh Journal of Veterinary Medicine, 16(1), 121-129.
- Alvarez-Pellitero, M., Simón Vicente, F., & González Lanza, C. (1981). Nuevas aportaciones sobre Dactylogyridae (Monogenea) de la cuenca del Duero (NO. de España), con descripción de Dactylogyrus polylepidis n. sp. y D. bocageii n. sp.
- Gonzalez-Lanza, M. C., & Alvarez-Pellitero, M. P. (1982). Description and population dynamics of Dactylogyrus legionensis n. sp. from Barbus barbus bocagei Steind. Journal of Helminthology, 56(3), 263-273.
- Reed, P., Francis-Floyd, R., Klinger, R., & Petty, D. (2009). Monogenean parasites of fish. Fisheries and aquatic sciences. University of Florida UF, IFAS Extension. FA28, USA, 4, 1-4.
- Zoral, M. A., Futami, K., Endo, M., Maita, M., & Katagiri, T. (2017). Anthelmintic activity of Rosmarinus officinalis against Dactylogyrus minutus (Monogenea) infections in Cyprinus carpio. Veterinary parasitology, 247, 1-6.
- Zhang, X. P., Li, W. X., Ai, T. S., Zou, H., Wu, S. G., & Wang, G. T. (2014). The efficacy of four common anthelmintic drugs and traditional Chinese medicinal plant extracts to control Dactylogyrus vastator (Monogenea). Aquaculture, 420, 302-307.
- Borji, H., Naghibi, A., Nasiri, M. R., & Ahmadi, A. (2012). Identification of Dactylogyrus spp. and other parasites of common carp in northeast Iran. Journal of Parasitic Diseases, 36, 234-238.08333
- Koyee, Q. M. K., & Abdullah, S. M. A. (2019). Phylogenetic and secondary RNA structure analysis of monogenean gill ectoparasites(Dactylogyrus spp.) parasitizing certain freshwater fishes. Polish journal of veterinary sciences, 22(4), 667–675. https://doi.org/10.24425/pjvs.2019.129979