Ceratomyxosis is caused by Ceratomyxa Shasta which is a tissue-invading protozoan parasite. It is capable of causing serious infection in both adult and juvenile Salmonids.
The only known method of initiating an infection with C. Shasta is by exposure of fish to water containing the infectious stage. Since the disease has never been detected outside the Pacific Northwest, every effort should be made to contain it within its present range.
Scientific classification
Kingdom: Animalia
Phylum: Cnidaria
Class: Myxosporea
Order: Bivalvulida
Family: Ceratomyxidae
Genus: Ceratonova
Species: C. shasta
Causative agent
Ceratomyxa shasta is a myxozoan parasite that infects the intestinal tract of salmonids, including salmon, trout, and steelhead. This parasite has a spore-like structure, which allows it to survive in the environment for extended periods.
The spore contains four polar capsules, each of which contains a coiled filament that can be rapidly ejected upon contact with the host. This filament contains toxins that paralyze the host’s gut muscles and enable the parasite to penetrate the intestinal wall.
Laboratory experiments were conducted to determine the infective dose for three fish species: rainbow trout, Chinook salmon, and coho salmon. A single actinospore was enough to cause a fatal infection in susceptible rainbow trout. Increasing parasite concentration resulted in higher infection prevalence and mortality.
Large and small rainbow trout had similar infection prevalence and mortality rates. Chinook salmon did not become infected, even when challenged with 5000 actinospores.
Coho salmon had one fatal infection after being challenged with 1000 actinospores. The study confirmed that low doses of C. shasta can cause severe infection in highly susceptible fish and demonstrated that parasite concentration influences infection prevalence.
Morphology of the parasite
The Ceratomyxa shasta parasite has a complex life cycle that involves two hosts. The spores of the parasite are released into the water, where they can remain for up to a year.
When the spores are ingested by a suitable host, they release their filament, which penetrates the intestinal wall and releases the parasite’s infective stage, known as a triactinomyxon.
This stage is characterized by a long thread-like body with three tails, which allow it to move through the water and seek out its next host.
Once the triactinomyxon finds a suitable host, it attaches to the fish’s gills and penetrates the tissue, where it transforms into a myxospore. This stage is spherical and has two valves that open to release the parasites inside.
The myxospore then develops into a sporoplasm, which divides and gives rise to numerous small cells that migrate to the intestine, where they attach and grow into mature parasites.
The life cycle of the parasite
● The life cycle of Ceratomyxa shasta involves two hosts: a fish host and a freshwater bryozoan.
● The parasite’s spores are released into the water and can remain infectious for up to a year.
● When a fish ingests an infected bryozoan, the parasite’s spores are released into the fish’s gut.
● The spores then attach to the fish’s gut wall and release their infective stage, the triactinomyxon, which penetrates the fish’s gut and moves through the water to seek out the next host.
● The triactinomyxon attaches to the gills of a suitable host and penetrates the tissue, where it transforms into a myxospore.
● The myxospore develops into a sporoplasm, which migrates to the intestine, where it attaches and grows into mature parasites.
● These parasites release their spores into the water, where they can infect a bryozoan, completing the parasite’s life cycle.
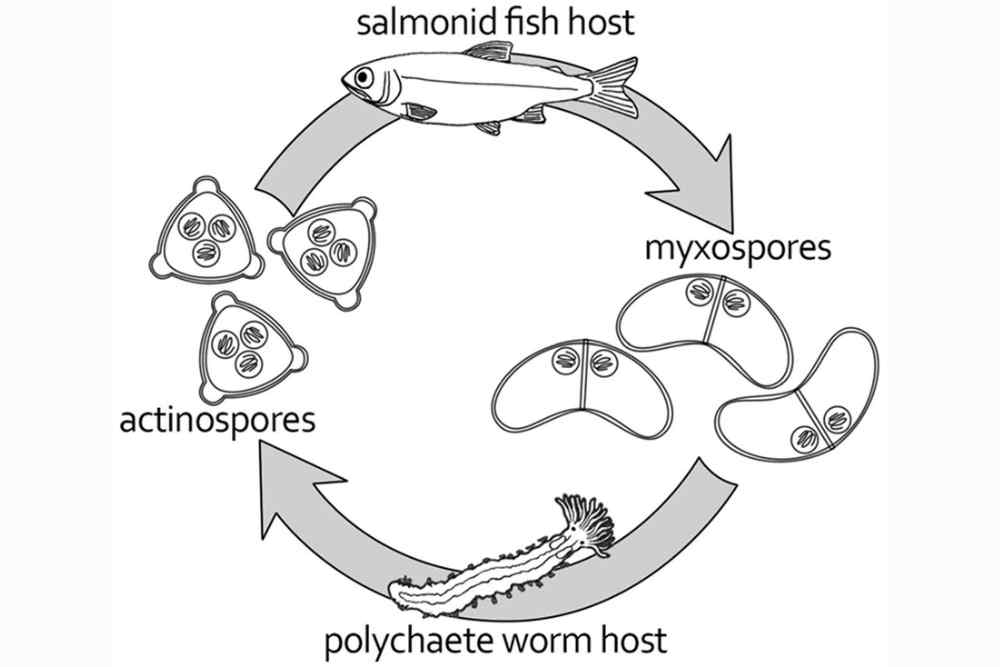
Figure: Life cycle of Ceratomyxosa Shasta; Source: ResearchGate
In a Laboratory study, a susceptible strain of rainbow trout and a resistant strain of Chinook salmon were exposed to the parasite to investigate differences in infection progress.
The gills were found to be the site of attachment and invasion of the parasite, which then migrated to the blood vessels and replicated. Parasite levels increased in the blood and coincided with host mortality.
The timing of parasite replication and migration to the intestine were similar for both species. The high exposure dose apparently overwhelmed the Chinook salmon’s defenses, as no evidence of resistance was observed.
Geographical distribution of Ceratomyxa shasta
Ceratomyxa shasta is a myxozoan parasite that infects salmonid fish, particularly Chinook salmon and rainbow trout, in freshwater environments of the Pacific Northwest region of North America.
It is found in rivers and streams along the Pacific coast from Alaska to California, as well as in some inland waterways of the region.
The parasite has been reported in several rivers and streams, including the Klamath, Trinity, Sacramento, Rogue, Umpqua, and Columbia Rivers, among others.
It is most prevalent in the Klamath River Basin, where it has caused significant mortality in Chinook salmon populations.
Overall, the distribution of Ceratomyxa shasta is limited to the Pacific Northwest region of North America and is largely dependent on the distribution of its fish hosts and suitable environmental conditions for its life cycle.
A survey was conducted from 2003 to 2005 to study the occurrence and relative abundance of the freshwater polychaete host, Manayunkia speciosa, for the myxozoan parasite Ceratomyxa shasta in the Klamath River. The survey identified populations of M. speciosa in pools, eddy-pools, and runs throughout the river, with large populations at the inflow to main-stem reservoirs.
The prevalence of C. shasta within selected polychaete populations was estimated to be 0.27%, with an area of elevated infection prevalence (4.9 and 8.3%) identified in 2 populations below a barrier to salmonid migration, explaining the high infectious spore densities demonstrated in concurrent studies and observations of C. shasta-induced mortality in Klamath River fall Chinook salmon.
Fish species affected by Ceratomyxa shasta
Ceratomyxa shasta is a myxosporean parasite that infects salmonid fish species. It is known to affect a variety of salmonid species, including:
- Chinook salmon (Oncorhynchus tshawytscha)
- Coho salmon (Oncorhynchus kisutch)
- Steelhead (Oncorhynchus mykiss)
- Rainbow trout (Oncorhynchus mykiss)
- Cutthroat trout (Oncorhynchus clarkii)
- Brown trout (Salmo trutta)
- Brook trout (Salvelinus fontinalis)
- Bull trout (Salvelinus confluentus)
Ceratomyxa shasta can cause severe infections in these fish, leading to a disease known as ceratomyxosis. The disease can result in high mortality rates, especially in juvenile fish.
Symptoms
Ceratomyxosis is a disease caused by the parasite Ceratomyxa shasta, which infects salmonid fish species, such as salmon and trout. The signs and symptoms of ceratomyxosis can vary depending on the severity of the infection, but commonly include:
Behavioral changes: infected fish may show abnormal swimming behaviour, such as swimming near the surface, darting, or spinning in circles.
Physical changes: the skin of infected fish may appear darker or redder than normal, and they may have visible lesions or sores on their skin, fins, or gills.
Respiratory problems: infected fish may show signs of respiratory distress, such as gasping for air or rapid breathing.
Loss of appetite: infected fish may refuse to eat or show a reduced appetite.
Weight loss: infected fish may lose weight due to reduced appetite or the parasitic infection consuming nutrients.
Death: severe cases of ceratomyxosis can lead to the death of infected fish, particularly if they have weakened immune systems due to other stressors such as water temperature and quality, which can lead to secondary infections.
Diagnosis
The diagnosis of ceratomyxosis is usually based on a combination of clinical signs, laboratory testing, and examination of affected fish. Here are some of the diagnostic methods commonly used:
Clinical signs: As described in the previous answer, fish affected with ceratomyxosis exhibit a variety of behavioural and physical changes that may indicate the presence of the disease.
Microscopic examination: Examination of fish tissues or smears under a microscope may reveal the presence of the parasite Ceratomyxa shasta, which is characterized by its spores.
Polymerase chain reaction (PCR): PCR is a molecular technique that can be used to detect the presence of Ceratomyxa shasta DNA in fish tissues or water samples.
Histopathology: Histopathology is the examination of tissues under a microscope to identify any changes or abnormalities. In fish with ceratomyxosis, histopathology may reveal inflammation or lesions in the gills or other tissues.
Serology: Serology involves testing blood or other fluids for the presence of antibodies against Ceratomyxa shasta, which can indicate a previous or current infection.
Prevention
Preventing the spread of Ceratomyxa shasta, the parasite that causes ceratomyxosis, is critical in maintaining the health of salmonid fish populations. Here are some measures that can be taken to prevent the spread of the disease:
Quarantine new fish: New fish, especially those from unknown sources, should be quarantined for at least two weeks in a separate tank or pond to ensure they are free of the parasite.
Use certified disease-free fish: Use fish that have been certified free of Ceratomyxa shasta or other diseases to reduce the risk of introducing the parasite to a new environment.
Minimize stress: Environmental stressors such as poor water quality, crowding, or changes in water temperature can weaken the immune system of fish and make them more susceptible to infections, including ceratomyxosis. Therefore, minimizing stressors can help prevent the disease.
Proper sanitation: Proper sanitation and hygiene practices, such as disinfecting tanks, nets, and other equipment, can help reduce the risk of spreading the parasite.
5.Use of medication: Chemical treatments or medications can be used to control the spread of ceratomyxosis in fish populations. However, care should be taken to ensure that the treatment does not harm other aquatic organisms or the environment.
Fish stocking strategies: Stocking fish at different times of the year, or introducing fish species that are not susceptible to ceratomyxosis, can help prevent the spread of the disease.
Treatment Strategies
Chemical treatment: Chemical treatment is one of the strategies used to control the spread of ceratomyxosis in fish populations. Here are some of the commonly used chemicals for the treatment of ceratomyxosis:
Formalin: Formalin is a chemical that is commonly used to treat fish infected with Ceratomyxa shasta. It is usually administered through the water and can be toxic to other aquatic organisms if not used properly.
Chloramine-T: Chloramine-T is another chemical used to treat fish infected with Ceratomyxa shasta. It is also administered through the water and is generally considered less toxic than formalin.
Copper sulfate: Copper sulfate is a chemical that is toxic to the parasite Ceratomyxa shasta. It can be used as a bath treatment or as a medication administered through fish feed.
Potassium permanganate: Potassium permanganate is a chemical that can be used to treat fish infected with Ceratomyxa shasta. It is usually administered through the water and can be toxic to other aquatic organisms if not used properly.
Biological treatment
There are several biological treatment methods for ceratomyxosis, including:
Parasite-specific drugs: Some drugs can be used to specifically target the Ceratomyxa shasta parasite. These drugs are usually administered to the fish through water or through injection.
Immune system boosters: Boosting the immune system of the infected fish can help them fight off the parasite. This can be done by feeding them immune-boosting supplements or by adding these supplements to the water.
A study aimed to understand the immune response of resistant steelhead trout to the parasite Ceratonova shasta, which causes severe inflammation in the intestine.
The results show that resistant fish rapidly induce immune factors and tissue responses that limit the spread of the parasite and subsequent tissue damage, enabling them to recover from the infection.
This provides a framework for future studies on the infection dynamics of C. shasta and other myxozoans.
Probiotics: Probiotics are beneficial bacteria that can be added to the fish’s diet or water. These bacteria can help improve the fish’s immune system and reduce the severity of the infection.
Vaccines: Researchers are developing vaccines that can help protect fish from Ceratomyxa shasta infection. These vaccines work by stimulating the fish’s immune system to produce antibodies that can recognize and fight off the parasite.
Genetic selection: Some fish populations may be naturally resistant to Ceratomyxa shasta infection. Selective breeding programs can be used to identify and breed fish with this resistance, which can help reduce the impact of the disease in aquaculture settings.
However, previous studies suggested that resistance to C. shasta is genetically controlled. But a study found that resistance was inherited as a single Mendelian locus, but was polygenic in nature, involving multiple genomic loci.
The study used genetic markers to determine the number and potential locations of these loci contributing to variation in survival phenotypes of doubled-haploid progeny.
It’s important to note that the effectiveness of these treatments may vary depending on the severity of the infection, the species of fish affected, and other factors. It’s always best to consult with a veterinarian or fish health expert before attempting to treat a fish disease.
Physical treatment
In addition to biological treatment methods, there are also several physical treatment methods for ceratomyxosis. These methods include:
Water management: The Ceratomyxa shasta parasite requires specific water conditions to survive and reproduce. Modifying the water temperature, flow rate, or oxygen levels can help reduce the parasite’s survival and growth. This can be done by adjusting the flow rate of the water or by adding oxygen to the water.
Ultraviolet (UV) light treatment: UV light is effective in killing parasites and bacteria in water. UV light can be used to treat the water in fish tanks or ponds, which can help reduce the number of Ceratomyxa shasta parasites in the water.
Mechanical removal: Ceratomyxa shasta parasites can attach themselves to the skin, gills, and intestines of fish. Mechanical removal involves physically removing these parasites from the fish using methods such as hand stripping or using a net to remove the fish from the water and manually removing the parasites.
Reference
- Bartholomew, J. L. (2012). 3.2. 4 Salmonid Ceratomyxosis. 3.2. 4 Salmonid Ceratomyxosis.
- Hendrickson, G. L. (1986). Ceratomyxa shasta: Geographic and Seasonal Distribution, Salmon Strain Susceptibility. University of California Sea Grant College Program Annual Report, 69.
3.Ibarra, A. M., Gall, G. A. E., & Hedrick, R. P. (1990). Trials with fumagillin DCH and malachite green to control ceratomyxosis in rainbow trout (Oncorhynchus mykiss). Fish Pathology, 25(4), 217-223. - Yokoyama, H., Grabner, D., & Shirakashi, S. (2012). Transmission biology of the Myxozoa. Health and environment in aquaculture, 3-42.
- Stocking, R. W., & Bartholomew, J. L. (2004). Assessing links between water quality, river health and Ceratomyxosis of salmonids in the Klamath River system. Department of Microbiology, Oregon State University, Corvallis, OR, 81.
- Ray, R. A., Rossignol, P. A., & Bartholomew, J. L. (2010). Mortality threshold for juvenile Chinook salmon Oncorhynchus tshawytscha in an epidemiological model of Ceratomyxa shasta. Diseases of aquatic organisms, 93(1), 63–70. https://doi.org/10.3354/dao02281